The cause of traumatic haemorrhagic shock, resulting from acute haemorrhage with soft tissue injury and the immune response, is a critical decrease in circulating plasma volume [1]. The effects of shock are reversible in the early stages, but they can lead to multiple organ failure and death if not treated early [2].
Inflammation and apoptosis are the 2 main factors in the progression of haemorrhagic shock. There is a positive correlation between the severity of the inflammatory condition and mortality [3]. It is known that histopathological changes develop in the lung as a result of infiltration by inflammatory cells [4]. It has been stated that there is still debate about how optimal fluid therapy should be performed and which fluid type might be advantageous in patients with lung injuries requiring thoracic surgery [5]. Trauma-related coagulopathy is a condition in which complex haemostatic and immunoinflammatory responses cause abnormal clot formation and activation of anticoagulant pathways rather than procoagulants [6]. Bedside viscoelastic coagulation tests provide information on clot dynamics and quality [7]. Thromboelastography (TEG), used for this purpose, evaluates all thrombin- mediated processes.
Haemodynamic compensatory mechanisms maintain vital organ perfusion in case of volume loss of up to approximately 30% of total blood volume [8]. Mild to moderate blood loss can be managed with crystalloid alone or colloid infusions. How-ever, there is a gap in the knowledge about how the blood transfusion should be done, and how and which type of crystalloid and colloid fluids should be used until the blood and blood products are provided are still controversial. A study by Hilbert-Carius et al. [9] reported that synthetic colloid resuscitation does not have any beneficial effects and might be harmful in patients with severe trauma. Moreover, the use of hydroxyethyl starch (HES) has been associated with a decrease in the levels of circulating factor VIII and von Willebrand factor, deterioration in platelet function, and prolongation of thromboplastin and activated partial thromboplastin time [10].
This experimental study aimed to monitor with systematic and quantitative methods the haemostatic and haemodynamic effects of acute traumatic coagulopathy due to resuscitation with different colloid fluids to reveal the endothelial tissue damage with histopathological data in the model of haemorrhagic shock induced in rats. Our hypothesis in this study was that colloid fluids could play an active role in the replacement of intravascular volume, optimise haemodynamics, and contribute to the improvement of endotheliopathy in the lung tissue, but increase coagulation disorders.
MATERIAL AND METHODS
Animals and experimental protocol
Following the Institutional Animal Experimentation Ethics Committee approval (protocol number: 2019-06/03), a haemorrhagic shock model was formed with injury in 20 male 6-month-old Sprague-Dawley rats, with 5 rats in each group. At least 5 days prior to the experiment, 2 to 5 male rats per cage were allowed to have free access to food and water, followed by a 12-hour light-dark cycle at 24°C ± 1°C and 55% ± 5% humidity. Animals fasted 12 hours before the surgery. They were anaesthetized by intraperitoneal injection of a combination of ketamine hydrochloride (90 mg kg–1) + xylazine hydrochloride (10 mg kg–1). Thermal blankets were used to prevent heat loss in the animals. The animals that did not undergo haemorrhage were used as the sham group (group S). The others were randomised into 3 groups to have haemorrhage and intravenous 0.9% NaCl as a replacement fluid (group C: control group), haemorrhage and gelatine (Gelofusine®, B. Braun Melsungen AG, Melsungen, Germany) infusion as a replacement fluid (group G), and haemorrhage and HES (HES, B. Braun Medical, Melsungen, Germany) infusion as a replacement fluid (Group V). Gelatine required 0.04 g polygeline in 500 mL, HES was 130/0.4 in 500 mL, and a 6% concentration was used.
Monitorisation
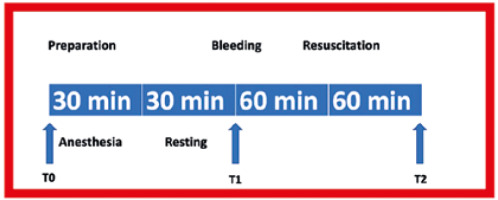
Blood pressure, respiratory rate, and heart rate were monitored using an electrophysiological data acquisition system (PowerLab 16/35, AD Instruments, Castle Hill, Australia). Mean blood pressure was measured at the carotid artery with a 24G catheter using a reusable BP transducer (MLT0380/D, AD Instruments, Castle Hill, Australia). The carotid artery was also used to collect blood samples and to create a haemorrhage. The tail vein was cannulated for intravenous fluid infusion. Respiratory rate and heart rate were measured non-invasively and recorded (Figure 1). The respiration rate was calculated using PowerLab-connected pulse transducers (TN1012/ST, AD Instruments, Castle Hill, Australia). Heart rate was measured using PowerLab-connected biological amplifiers (Bio Amp FE231, AD Instruments, Castle Hill, Australia).
Haemorrhagic shock protocol
Following the preparation and anaesthesia period (T0), the target blood volume loss for haemorrhage was determined as 40%. A double-lumen catheter was used for the experiment. While blood was drawn from one lumen with a syringe, a sterile sodium citrate solution of 4% in 250 mL was administered through the other lumen following 1 : 9 dilution. The procedure was interrupted if the mean arterial pressure fell below 40 mmHg. Later, haemorrhage was continued when a pressure of 45 mmHg was maintained for more than 15 seconds. The total blood of a rat with an average weight of 250 g is 16 mL. Studies have shown that removing more than 15% of blood volume can cause hypovolaemic shock [11]. Haemorrhage was achieved in 3 stages: in the first stage, a loss of 25% was ensured with haemorrhage at a rate of 0.5 mL min–1. In the second stage, 10% blood loss was achieved at a rate of 0.2 mL min–1. In the third and final stage, 5% blood loss was achieved at a rate of 0.1 mL min–1. In the first stage, a blood sample was taken (T1). Resuscitation fluid was administered to the rats for 60 minutes as an IV infusion, and the last blood sample was collected at the end of 60 minutes (T2) [12, 13]. After that, the vascular catheters were withdrawn, the incisions were closed, the rats were sacrificed, and tissue samples were taken (Figure 2). Euthanasia was performed by decapitation and cervical dislocation [14].
The rats were randomised, and the colloid fluids were infused intravenously. The volume of HES infused was equal to the lost blood volume, and the volume of gelatine infused was 1.5 times higher than the lost blood volume. Resuscitation fluids were heated up to 37°C prior to infusion and infused within 60 minutes.
Viscoelastic test measurement
A TEG (Model 5000, Hemoscope Corporation, Niles, IL) device was used for analysis. The TEG was used to measure the time from the onset of coagulation to thrombus formation (R value min–1), the time from clot formation to an amplitude of 20 mm (K value min–1), the rate of clot reaching its maximum power (ANGEL/alpha angle/α), maximum amplitude or firmness of the clot (MA mm–1), and clot stability-lysis (LY30/%). The blood samples were collected in tubes that contained 3.8% sodium citrate. The sitting time between collection and test initiation was determined up to 2 hours. Kaolin was used as an activator. Standardisation began with the collection of samples. The ability to reproduce a test was revealed using standardised preanalytical procedures in thromboelastogram [15]. TEG values were studied in the T2 time frame, i.e. when bleeding and then resuscitation was completed.
Annexin A5 measurement
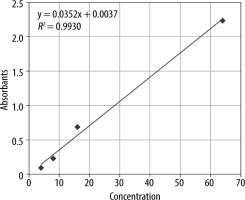
Rayto RT-6000 ELISA microplate reader and microplate washer Rayto devices were used for annexin A5 measurements made with ELISA. Blood samples were collected before bleeding and after resuscitation for annexin A5 values. To obtain serum, 5-mL blood samples were taken in a tube containing vacutainer serum separator gel, and the tubes were kept at room temperature and centrifuged at 100 g for 10 minutes within the first hour of blood collection. After separation, the serums were frozen at –20°C, and annexin A5 measurements were performed with ELISA kits (BIOSOURCE, Shangai, China). The microtiter plate was incubated, and then the plates were thoroughly washed to remove all unbound components. Substrate solutions were then added to each of them. HRP and substrate were allowed to react for a short incubation period. Only sections containing ANXV and enzyme-conjugated antibodies showed a change in colour. The enzyme-substrate reaction was terminated by adding a sulfuric acid solution, and the colour change was measured spectrophotometrically at 450 nm. The result was measured by absorbance (A450nm) in a microplate reader. An annexin A5 standard (0–10 ng mL–1) curve was plotted, and the data were calculated in units of ng/mL annexin A5. The calibration graph is shown in Figure 3.
Histopathological examination
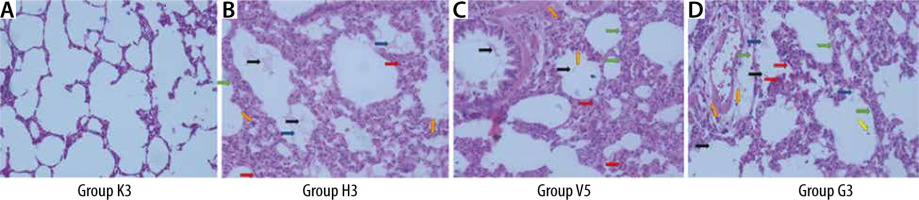
Lung tissues were taken for histopathological examination, and then the tissues were fixed with a 10% neutral buffered formalin solution. Lung tissue samples were processed using the Thermo Excelsior ES tissue processor and embedded in paraffin. Serial sections of 4-5-μm-thick paraffin blocks were obtained with Thermo microtome. Sections were stained with haematoxylin and eosin (H&E) for histopathological examinations. The sections were photographed with a Leica DM2000 light microscope and with the camera of an Apple iPhone X mobile phone. The sections were evaluated for neutrophils in the alveolar space and interstitial space, hyaline membrane, proteinaceous debris in the air spaces, alveolar septal thickness, and haemorrhage (Table 1) [16].
TABLE 1
Scoring system used in lung injury assessment
The lung damage score was calculated with the following formula:
Lung injury score = [(20 × A) + (14 × B) + (7 × C) + (7 × D) + (2 × E)]/(number of examined areas × 100),
where A is neutrophils in the alveolar space, B is neutrophils in the interstitial space, C is hyaline membranes, D is proteinaceous debris filling the airspaces, and E is alveolar septal thickening.
Statistical analysis
IBM SPSS Statistics 22 was used for statistical analysis. We used the mean, standard deviation, median, minimum, maximum, frequency, and ratio values for descriptive statistics. The Kolmogorov-Smirnov test was used to measure the distribution of the variables. The one-way ANOVA test was used for normally distributed numerical data, and the Kruskal-Wallis H test was used for non-normally distributed numerical data. Pearson’s correlation analysis was used to determine the relationship between quantitative variables. The results were evaluated with a 95% confidence interval and with a significance level of P < 0.05. Values are represented as mean ± standard error.
RESULTS
TEG analysis
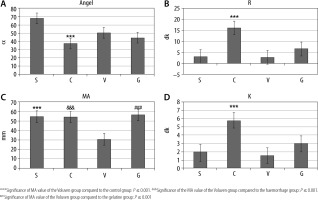
The R and K values were statistically significantly higher in group C than in the other groups. The ANGEL angle value in group S was statistically significantly higher than that of group C (P < 0.05). The MA value in group V was statistically significantly lower compared to the other groups (P = 0.001) (Table 2, Figure 4).
TABLE 2
TEG analysis
Respiratory rate analysis
Respiratory rate values at the zeroth minute of haemorrhage were statistically significantly higher in group G than the values in group S (P < 0.05). At the 15th minute of infusion, the respiratory rate was found to be significantly higher in group G compared to the control group (P < 0.05).
Heart rate analysis
The heart rate values at the 15th and 30th minutes of the infusion were statistically significantly lower in group C than in group S (P < 0.05).
Blood pressure analysis
Blood pressure values at the 60th minute of haemorrhage were found to be significantly lower in group C compared to group S (**P ≤ 0.01). At the 60th minute of haemorrhage, the blood pressure value of group G was found to be significantly higher than that of group C (##P ≤ 0.01). Blood pressure values at the 0th, 10th, 15th, 30th, and 60th minutes of infusion were statistically significantly lower in group C than in group S (**P ≤ 0.01). On the other hand, blood pressure values at the 0th, 10th, 15th, 30th, and 60th minutes of infusion were statistically significantly higher in group G and V compared to group C (##P ≤ 0.01, ddP ≤ 0.01) (Table 3).
TABLE 3
Blood pressure analysis
Annexin A5 analysis
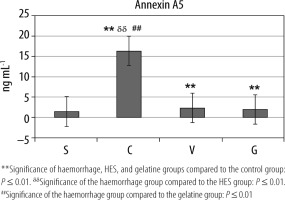
Compared to group S, annexin A5 values were statistically significantly higher in groups C, V, and G (**P ≤ 0.01). Moreover, annexin A5 value was the highest in group C (ddP ≤ 0.01, ##P ≤ 0.01) (Table 4, Figure 5).
Analysis of lung injury scores
The lung damage scores in the C, V, and G groups were statistically significantly higher than the lung damage scores in group S (**P ≤ 0.01). Also, the lung damage score in group C was statistically significantly higher than the lung injury scores of group V and group G (ddP ≤ 0.01, ##P ≤ 0.01) (Table 5, Figure 6).
Correlation analysis
A positive correlation was observed between haemorrhage scores, lung injury scores, and TEG R and K (P < 0.05) (Table 6). There was a positive correlation between lung injury score and TEG R and K, and annexin A5 apoptosis assay (P < 0.05). A negative correlation was observed between ANGEL angle, blood pressure at the 0th and 60th minutes of haemorrhage, and blood pressure values at the 0th, 10th, 15th, 30th, and 60th minutes of infusion (P < 0.05).
TABLE 6
Correlation analysis
DISCUSSION
It was indicated in our study, in which a haemorrhagic shock model was generated, that a 6% HES solution used as resuscitation fluid affected TEG coagulation parameters more than gelatine fluid did. We determined that resuscitation with gelatine fluid had a more positive effect on coagulation. It was also demonstrated that resuscitation with both colloid fluids had a positive effect on haemodynamics.
In a study comparing different resuscitation fluids and including 100 trauma patients, there was a significant prolongation of prothrombin time (PT) and International Normalized Ratio (INR) levels compared to baseline values in the groups receiving HES and gelatine [17]. In this study, the significant increase in R value observed in the haemorrhage group compared to the control group indicates a delay in the onset of enzymatic clot formation. However, there was no significant difference between the R values of the control, HES, and gelatine groups. This indicates that HES and gelatine have similar effects on haemodilution and coagulation factors in traumatic haemorrhagic shock. The effects of HES and gelatine on TEG parameters were investigated in patients who had major orthopaedic surgery but did not develop haemorrhagic shock [18]. In that study, no difference was found in the R, K, and alpha angle values in the HES group, but a decrease was noted in the MA values. It was also reported in the same study that although there was a significant increase in R time in the group given gelatine, the K time, α angle, and MA values did not change significantly in that group. In our study, the alpha angle was decreased in all groups, but this decrease was significant in the haemorrhage group. On the other hand, a study comparing TEG values of pregnant women who received HES and gelatine loading prior to caesarean section reported that the alpha angle lowered significantly with HES, but gelatine did not affect this value [19]. This result indicates that trauma and accompanying haemorrhagic shock may have different effects on coagulation parameters assessed by TEG. In the present study, there was a significant decrease in the MA of the group receiving HES, resulting in a decrease in clot amplitude and a delay in coagulation. Similarly, several studies have reported the same results. A study in which blood samples collected from 22 healthy dogs after HES infusion were subjected to TEG analysis reported a decrease in both MA levels and alpha angle [20]. However, it was emphasised that this decrease did not have a significant impact on haemodilution. In an in vitro experimental study that included 6 healthy dogs, MA was significantly extended with HES [21]. On the other hand, the effects of HES were compared with those of 0.9% NaCl on 21 dogs, and it was emphasised that the HES solution had significant negative effects on clotting time and clot strength [22]. It was reported in a systematic review of HES, in which data from 17 in vitro and 7 in vivo studies were analysed, that HES adversely affected clot formation due to hypo-coagulative effects [23]. Because HES molecules diminish platelet reactivity by blocking the access of ligands to surface receptors through the conformational change of GP IIb-IIIa, negative effects may have been detected in the MA value, which represents the clot strength and especially optimised by platelets [24]. Additionally, a decrease in von Wille-brand factor (VWF) and factor VIII occurs [25]. A dilutional coagulopathy is present, leading to acquired fibrinogen dysfunction or deficiency [26].
The lung damage score was found to be significantly higher in all study groups when compared to the control group. Furthermore, a positive correlation was observed between lung injury score and TEG R and K, and annexin A5. It was demonstrated that the lung injury score in the groups treated with the infusion of both HES and gelatine was significantly lower than the group that had haemorrhage and did not receive colloids. It was found in a study that patients treated with HES infusion had better perioperative and postoperative pulmonary functions compared to gelatine-treated patients [27]. Better gas exchange, higher respiratory compliance, and lower lung injury scores were reported in HES-treated patients compared to those treated with gelatine infusion. However, although lung damage was revealed in our study, no significant difference was found between the 2 colloids, which may be due to the limited sample size. In a porcine haemorrhagic shock model, HES, gelatine, and balanced electrolyte solution were compared [28]. This study revealed that the colloids, similarly to our study, stabilised the macro-circulation; however, the coagulation system was significantly affected.
In our study, the blood pressure values of rats subjected to haemorrhagic shock fell significantly following the haemorrhage. A concurrent decrease in heart rate in the haemorrhage group indicates cardiovascular depression. This indicates that the haemorrhagic shock model was applied appropriately, and it also reveals the treatment response significantly. The elevation in blood pressure of the groups treated with colloids reveals the positive effect of the treatment, but the fall in blood pressure compared to the control group is still remarkable. Effects on renal histology [29], viscoelastic coagulation [30], lung and renal injury [31], pulmonary histology [32], or apoptosis and tissue oxidative stress [33] have been investigated in the literature by creating different traumatic haemorrhagic shock models. However, a limited number of studies have investigated the effects of different colloids on microcirculation and the viscoelastic components of blood together. Therefore, we believe that this study will be useful and contribute to the literature regarding the issue of providing microcirculation preservation with resuscitation in the early period, which is still controversial.
Circulating annexin V is an anticoagulant protein. In our study, we detected a significant decrease in annexin A5 levels in rats that received either HES or gelatine solution. However, this decrease was found to be significantly higher in the gelatine group when compared to the HES group. It has been shown that the negative contribution of gelatine to annexin A5 is less than that of HES. Additionally, it has long been known that the molecules in HES fluids bind to the adhesion receptors of platelets and cause negative effects on coagulation in the first step of haemostasis. Although some studies did not identify any difference between the effects of gelatine and HES fluids on inflammation, there are resources mentioning the negative effects of HES fluids on organ dysfunction and endothelium [34]. Different effects of colloid fluids should be taken into consideration in the selection of resuscitation fluids in the case of a large volume deficit. However, further clinical studies in which microcirculation can be monitored should be conducted to further define the importance of different resuscitation fluids for haemostasis, apoptosis, and lung tissue.
LIMITATIONS
The lack of a whole blood replacement group is a substantial limitation. Another limitation is that the devices that were planned to be used for microcirculation measurements during the project designing phase of the study could not be accessed because of the travel restrictions implemented due to the COVID-19 pandemic.
CONCLUSIONS
Lung tissue damage occurs in haemorrhagic shock. Simultaneously, there is a delay in the onset of coagulation, thrombus formation, and stable fibrin formation, resulting in a decrease in the rate of reaching maximum clot strength in the treatment groups. All these pathological events are positively impacted by fluid resuscitation with HES or gelatine. Due to the possible negative effect of HES on platelets, there was a decrease in the amplitude of the clot formed in this group. For this reason, it has been concluded that using gelatine may be advantageous in the process until blood transfusion is initiated in trauma.