Introduction
Acute myocardial infarction (AMI) remains one of the leading causes of death worldwide during cardiovascular diseases; nevertheless, AMI mortality has decreased due to comprehensive medical and interventional treatment and prevention [1]. Increased survival after AMI leads to an increase in the number of patients living with heart disease. Therefore, there is a need for optimal secondary prevention throughout their life [2]. An important step in the secondary prevention of recurrent myocardial infarction is cardiac rehabilitation (CR). The main goals of CR are to reverse the physiological and psychological effects of myocardial infarction, to achieve clinical stabilization (which leads to a significant reduction in hospitalizations, adverse events, and premature death), to optimize risk management, and to improve the psychosocial and psycho-emotional status of patients [3, 4]. However, with the onset of the global COVID-19 pandemic, the CR programs in many clinics were limited due to quarantine measures [5]. The COVID-19 pandemic has undeniably influenced CR efforts around the world [6–9]. There are heterogeneous data on the pathophysiology and morphology of myocardial damage during the COVID-19 pandemic [10, 11]. Rey et al. revealed the development of AMI due to thrombosis of both stenosed and unchanged coronary arteries [12]. In the study of Wang et al. an increased level of cardio-specific markers (troponin, NT-proBNP, etc.) was observed in 8% of patients with COVID-19 and was not accompanied by clinical deterioration [13]. The pandemic caused delays in reaching hospitals, door-to-lytic and door-to-balloon time, many patients refrained to call ambulance services when experiencing chest pain [14], all these factors contributed to complicated course of AMI and unfavourable outcomes. Mortality among patients with ST-segment elevation myocardial infarction (STEMI) more than tripled during pandemics, from 4% in 2019 to 14% during the outbreak, while the rate of post-AMI complications after intervention for STEMI increased from 10.6% to 19%. However, little is known about CR rehabilitation in the COVID era, when the course of AMI is complicated due to the above-mentioned factors and patients’ mobility is restricted due to quarantine [15]. Most researchers still emphasize the importance and necessity of rehabilitation measures that must continue despite restrictive measures during the pandemic [16]. In fact, multimodal CR programs have been shown to provide greater input for survival compared to exercise-only interventions. Moreover, most CR programs are not personalized to the preferences of individual patients or populations, especially when COVID-19 is present, which exacerbates the course of myocardial infarction [17]. Knowledge about the effects of CR on quality of life and exercise tolerance in AMI patients with COVID is scarce.
Aim
The aim of the study was to evaluate the use of a modular CR program on quality of life and exercise tolerance among post-infarction patients with COVID-19 recovery and those with no history of COVID-19 infection, and to explore the relationship between quality of life measures and exercise tolerance in COVID-19 patients with AMI.
Material and methods
The design of this study is a prospective cohort clinical study.
This study included 118 patients with history of AMI admitted to the rehabilitation department of the Cardiomed clinic through the portal of the hospitalization bureau, selected according to the inclusion and exclusion criteria.
Criteria for inclusion:
patients with a documented history of myocardial infarction, who had and had not undergone percutaneous coronary intervention (PCI) with coronary artery stenting.
Criteria for exclusion:
patients with open heart surgery (coronary artery bypass grafting, mammary coronary bypass grafting, correction of valvular defects, correction of ASD, VSD, etc.),
patients referred to the CR after stenting, but without myocardial infarction.
To evaluate the impact of a modular CR program among patients, they were divided into 2 groups: the first group included 86 patients who had “ground-glass opacity” changes in the lungs on computed tomography (CT), and the second group comprised 32 patients who had no history of coronavirus infection or no change on CT scan of the lungs during the pandemic. The study was conducted in the rehabilitation department of the hospital “CardioMed Clinic”, located in Shymkent, Kazakhstan.
Baseline variables
We assessed baseline clinical variables: age, gender, number of days after AMI-related discharge from the hospital, ventricular tachycardia during AMI, records in ambulatory prior to AMI, n (%), stage of rehabilitation, risk factors of coronary artery disease (i.e. arterial hypertension, diabetes, smoking, overweight/obesity), body mass index, coronary artery interventions, stenting of the coronary arteries, respiratory rate (RR), heart rate (HR), blood pressure (BP), and chronic heart failure with NYHA class. The COVID-19 diagnosis was established by polymerase chain reaction (PCR) testing, and all patients in group 1 underwent computed tomography; the severity of COVID-19-induced changes in lungs were defined by Li et al. [18].
The CR program
The modular CR program consists of 5 modules: the first module is an initial assessment to determine the individual needs of each patient in CR for the preparation of individual rehabilitation programs. Based on the initial assessment, in the second module, patients were educated on relevant topics about risk factors and adherence to dietary therapy, as well as talks and discussions on the underlying disease. The third module provided the correction and selection of drug therapy for each patient participating in the CR. The fourth module is a daily physical training regime with an individual approach, starting with small load exercises and with a gradual increase, taking into account the level of heart contraction (HR) rate and the subjective assessment of load exercises on the Borg scale. The physical training module consisted of physical exercises, including on an exercise bike and a treadmill. The increase in intensity during the program was calculated according to both the HR rate response and the Borg scale (from 0 to 10 on the Borg scale) [19]. The HR was constantly monitored during sessions using ECG monitoring along with the Borg scores. The target HR rate was calculated according to the Karvonen formula [20]. The fifth and final module consists of physiotherapy according to indications, and as prescribed by a physiotherapist. The patients were hospitalized in the department of rehabilitation 3 times for the 2nd and 3rd stages of the CR after AMI. The interval between the first, second, and third hospitalization in the department for each patient was 3 months. During the 3-month break, the patient performed the recommended physical activity. The duration of modular rehabilitation for each hospitalization was 14 days.
Quality of life
Life quality parameters in both groups were assessed using the Seattle Questionnaire before and after (2-week period) the application of the CR modular program, as well as 3 and 6 months after the application [21]. The Seattle Questionnaire consists of 19 questions combined into 5 scales: physical limitation (PL); angina stability (AS); angina frequency (AF); treatment satisfaction (TS); and disease perception (DP). Each scale is rated from 0 to 100 points, and a high number of points indicates less restriction of physical activity (PL), lower frequency (AF) and changes in symptoms of angina pectoris (AS), high satisfaction with treatment (TS), and high disease perception (DP), respectively.
Exercise tolerance parameters
Exercise tolerance was evaluated using the 6-minute walk test [22], and the exercise stress test on the treadmill was performed according to the standard Bruce protocol. We assessed total CR training duration in seconds, metabolic equivalent (MET) [23], maximal oxygen consumption (VO2max) [24], and HR in beats/min. The exercise parameters were evaluated before, at the end of 2-week CR training, and 3 and 6 months later.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Cardiomed Clinic (study protocol No. 003E 09/11/2019).
Statistical analysis
IBM SPSS Statistics version 19 was used for statistical processing. To assess the normality of the distribution of quantitative data, the Kolmogorov-Smirnov test and the Shapiro-Wilk test were used – the distribution of almost all variables was non-normal. Therefore, nonparametric tests were used to compare related and unrelated samples. The Mann-Whitney U test was used to compared variables between groups. The Friedman test for repeated measures was used to compare QoL and exercise tolerance with groups before, at the end, and after 3 months and 6 months. Kendall’s tau-b correlation coefficient was used to assess the relationship between QoL and exercise tolerance variables because the distribution of variables differed from normal. The significance level was accepted at p < 0.05.
Results
General characteristics of patients are described in Table I below.
Table I
General characteristics of patients
Both groups were comparable in terms of age, body mass index (BMI), RR, BP and HR, number of smokers, risk factors like arterial hypertension and diabetes, number of days after AMI and early post infarction complications. But in the first group there were slightly more men, but this was non-significant, and the number of patients who were at the 3rd stage of rehabilitation was slightly higher in group 1. Patients after AMI and COVID-19 had higher NYHA 3 class (p = 0.023). Both groups of patients did not differ by coronary intervention type and target vessels.
Tables II and III present comparative data on the effectiveness of the modular CR program between patients with an AMI and COVID-19 and an AMI without COVID-19, before and after the CR, as well as 3 and 6 months after the CR.
Table II
Performance indicators of the modular CR program: Seattle Questionnaire results [SAQ]
Table III
Performance indicators of the modular CR program: exercise tolerance parameters
Physical limitation increased groups 1 and group 2 in time (p = 0.014 and p = 0.002), but the difference between groups did not reach statistical significance.
Disease perception showed a trend to increase in both groups. Effect of CR on exercise tolerance (Table III).
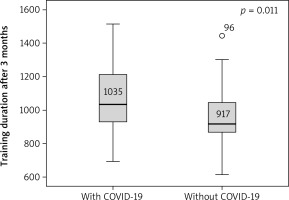
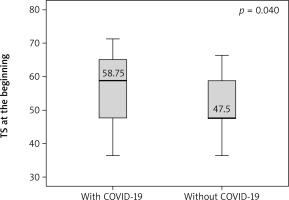
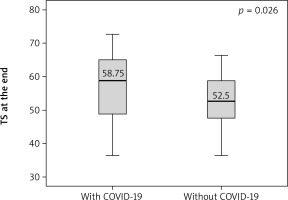
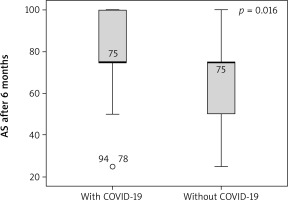
Angina stability improved in both groups (p = 0.046 and p = 0.001), and there were statistically significant differences between groups after 6 months (p = 0.016) (Figure 1). Angina frequency increased in thew group of AMI patients with COVID (p = 0.015) but did not change significantly in the group of patients with AMI only. Treatment satisfaction improved in both groups (p = 0.014 and p = 0.019); however, it was higher in patients with history of AMI and COVID-19 as compared to patients with AMI only at the beginning and the end of CR (p = 0.04 and p = 0.026) (Figures 2, 3). Both groups increased the MET significantly during the training period (p = 0.008 and p = 0.004) on a similar level. Similarly, maximum oxygen consumption during training increased in both groups from beginning to the 6th month (p = 0.002 and p = 0.008). Interestingly, the duration of training increased in both groups significantly (p = 0.008 and p = 0.008) but at a higher level at the 3rd month (p = 0.011) (Figure 4). The 6-minute walk test result increased in both groups significantly (p = 0.011 and p = 0.019).
The relationship between QoL and exercise tolerance parameters after CR
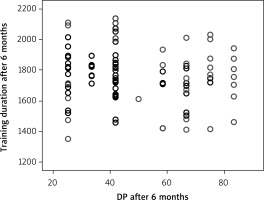
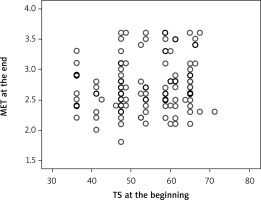
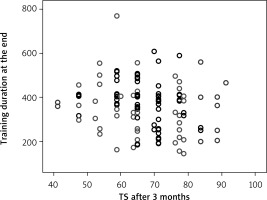
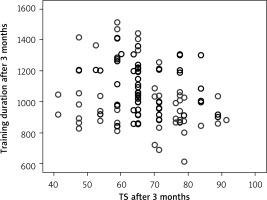
The quality of life parameters, like DP at the beginning of CR, had a direct correlation with MET at the end of the program (Figure 5) (R = 0.145, p = 0.042), and DP at the end of CR with MET at the end of CR (R = 0.151, p = 0.033) (Figure 6). A correlation remained between DP at the end of CR and MET at 3 months (R = 0.157, p = 0.027) (Figure 7). However, there was an inverse correlation of DP at 3 months after CR with training duration 6 months after CR (R = –0.140, p = 0.037) (Figure 8), and DP at 6 months after CR with training time after 6 months (R = –0.133, p = 0.048) (Figure 9). The treatment satisfaction (TS) of patients at the beginning of CR had a direct correlation with the MET parameter at the end of the CR (R = 0.133, p = 0.048) (Figure 10). However, there was also a negative association of TS at 3 months with the training duration at the end of the CR (R = –0.129, p = 0.048) (Figure 11), and after 3 months (R = –0.167, p = 0.010) (Figure 12). The TS at 6 months was associated with MOC at the end of CR (R = –0.148, p = 0.031) (Figure 13). The PL score at the beginning of CR was associated with the MET parameter after 6 months (R = 0.161, p = 0.017) (Figure 14).
Discussion
Our study demonstrated that the CR training program had a positive effect on quality of life and exercise tolerance in patients after AMI with and without COVID-19. Although initially patients with COVID-19 had higher NYHA functional class, all patients achieved the same level of QoL and exercise tolerance as patients without COVID-19. Treatment satisfaction even was higher in pts with COVID-19 at the beginning and the end of the CR program, which may be because they received more care because of COVID-19. Perception of physical limitations increased in patients with COVID after 6 months. This may be explained by the effect of long-covid syndrome, which might affect patients psychologically or neurologically [25]. Previous studies after 2 and 3 months of follow-up showed the persistence of the symptom of chest pain in 21.7%, 13.1%, and 12.7% of patients who had COVID-19, which indicates the consequences of post-covid syndrome [26–28]. The modular CR program pays attention not only to physical exercises (as in many recommendations), but also to adequate medication, reducing bad habits, developing a healthy lifestyle and diet, and limiting harmful transfats, all of which ultimately improves the quality of life. Results of the Seattle Questionnaire showed that in both groups physical tolerance increased, while more appropriate drug therapy was selected, the angina frequencies did not increase, and the results improved in patients who had AMI and COVID-19. The improvement in all variables was mainly at 3 months, and they were maintained during the 6-month follow-up period. When assessing the physical tolerance of patients, a gradual improvement in indicators for all points was revealed, the training duration in seconds was especially significantly increased in both groups, but in the group after MI it was better; however, by 6 months, patients who had had AMI and COVID-19 equalized. Taking into account the post-COVID condition in patients of the 2nd group, there is a decrease according to the results of the 6MWT. When evaluating the effect of physical activity parameters on quality-of-life indicators, it was determined that DP at the beginning of CR had a direct correlation with MET at the end of the program; however, an inverse correlation was found between DP 3–6 months after CR and training time 6 months after CR. The TS of patients at the beginning of the CR was directly correlated with the MET parameter at the end of the CR, indicating the importance of the modular CR program.
Modular CR programs include physical training and a correction of risk factors, nutrition, psychosocial counselling, and promotion of a healthy lifestyle. These exercise-based CR programs are effective in reducing morbidity, mortality, and readmission rates, and improving quality of life [29]. However, the effectiveness of CR programs and their attendance by patients has declined sharply during the global COVID-19 pandemic. At the same time, a study by O’Doherty et al. with the British Society for Cardiac Prevention and Rehabilitation identified the need for innovation in CR programs with patient-centred interactive technology resources that also contribute to a more pragmatic implementation of modular CR programs for patients after COVID-19 and their impact on participation and safety [30]. Based on the same recommendations, our modular CR program was developed and was then modified during the COVID-19 pandemic.
There is little information on the prognosis of AMI in patients with COVID-19. A recent series of studies by Bangalore et al. showed that half of the patients had coronary angiography and that one third of these patients had non-obstructive coronary artery disease. Myocardial injury in patients with COVID-19 may be multifactorial, including rupture of coronary plaques and microthrombi, cytokine storm, coronary spasm, endothelial injury, and myocarditis or taco-subocardiomyopathy [31]. In the study by Solano-López et al. more than two-thirds of COVID-19 patients with AMI died of ARDS or fulminant myocarditis; outcomes in these patients are determined by the severity of COVID-19 pneumonia and direct myocardial damage, while coronary artery thrombosis is more likely secondary [32]. For instance, Hendren et al. proposed the introduction of the concept of acute COVID-19 associated with cardiovascular syndrome (acute COVID-19 cardiovascular syndrome – ACovCS), which included a wide range of symptoms, including fatigue, chest pain, decreased exercise tolerance, cognitive impairment, shortness of breath, fever, headache, and loss of smell and taste, but palpitations are typical and atypical frequent complaints [33]. Heart rhythm disturbances occur in COVID-19 patients with fluctuations from 7.3% to 44% of patients [34]. This high prevalence of arrhythmia may be partly due to metabolic problems, hypoxia, or neurohormonal or inflammatory stress [35]. In our study, we found a minimum of 52 beats per minute and a maximum of 97 beats per minute, and tachycardia attacks prevailed, according to patients. Furthermore, because the average heart rate of 73 beats per minute was seen in both groups, we could not suppose that COVID-19 affects the feeling of heartbeat; however, it may also be associated with drug therapy. Thus, there is an urgent need to scale up and reorganize the CR and secondary prevention services. Recently, the working group on Cardiovascular Disease Prevention and Rehabilitation of the Dutch Society of Cardiology formulated practical recommendations for CR during the COVID-19 pandemic, in which the main proposals were shortening of CR programs, selection of the main components of exercises, initial thorough examination of the patient, post-COVID assessment of general condition and well-being, and compliance with all hygiene standards [17]. According to the recommendations of the Canadian Society of Rehabilitation, the use of modular CR programs based on physical training is justified and applicable even with tele-rehabilitation regimens that are also described in Japanese studies [36, 37].
Exercise can be an important way to rehabilitate patients with COVID-19. Exercise directly improves lung function and boosts immunity by correcting cytokine imbalances in the body. In addition, it reduces intracellular and extracellular oxidative stress. Another benefit of exercise is the regulation of gut flora homeostasis [38]. We offer a modular CR system that takes into account not only physical exercise, but also a medical approach with psychological exercises and physiotherapeutic procedures that, according to the results, showed treatment satisfaction among patients, as well as an increase in the duration of training (min. 216, max. 1789 sec). It is vital to consider the impact of CR on patients with COVID-19 in a comprehensive manner, and appropriate measures can reduce complications due to COVID-19 and provide a quick return to normal life. Future research will focus on specific types and methods of exercise for different exercise regimens.
With the recent success of COVID-19 vaccines, the burden of coronavirus will decrease, but a significant number of patients with persistent symptoms are likely to remain even months after exposure to COVID-19. As the medical community gains a better understanding of the pathophysiology of COVID-19 and its cardiovascular manifestations in the coming years, we hope to expand our ability to identify those at increased risk for these complications and discover effective strategies for prevention and treatment of this syndrome.
We did not assess the relationship of Qol and ET with severity, or outcomes of patients rehospitalization after a modular CR program in the emergency cardiology department; we also did not describe re-PCI rates, adequate medication influences, and arrhythmia disturbances.
Conclusions
The modular CR program improves exercise capacity and quality of life with AMI and COVID-19 similar to patients without COVID. COVID patients should subsequently undergo rehabilitation.