Purpose
Nasopharyngeal carcinoma (NPC) is more frequent in males, and within two age groups of 10-25 and 40-60 years old [1]. It is uncommon in western countries (1/100,000), but quite frequent in southern China (25-50/100,000) [2]. NPC incidence is not related to tobacco and alcohol habits, but to carcinogens exposure and Epstein-Barr virus infection [3]. The histology of most cases is squamous cell carcinomas or undifferentiated carcinoma, with high-risk of lymph nodal spread, which is often the first sign of the tumor.
The standard of care for advanced NPC in young patients with good performance status is EBRT combined with chemotherapy [4, 5]. Lin et al. reported better 5-year overall survival (OS) and progression-free survival rates with radio-chemotherapy (RCT) than with RT alone (72.3 and 71.6% vs. 54.2 and 53%, respectively, p < 0.01) [6].
Nevertheless, despite RCT, local recurrence occurs in up to 10-50% patients [7, 8]. Increasing radiation dose to primary tumor might allow improved local control (LC). Such a dose escalation can be achieved with the use of various radiotherapy techniques, such as three-dimensional conformal radiotherapy (3D-RT), intensity-modulated radiotherapy (IMRT), and IRT. IRT can be used either in case of local NPC recurrence/persistence, or as a boost after EBRT [9, 10]. Whereas the role of IRT in case of relapse/persistence has been well-established [11], the benefit of an IRT-based boost remain a matter of debate. Therefore, the aim of this systematic review was to analyze the efficacy of IRT as a boost after EBRT in patients with NPC in terms of LC, OS, disease-free survival (DFS), cancer-specific survival (CSS), and toxicity.
Material and methods
Development of clinical questions
Clinical question was formulated according to the PICO criteria as follow:
Identification of outcomes
Primary outcome was LC of the disease, which was defined as the absence of disease progression within the irradiated volume. Secondary outcomes were OS, DFS, CSS, and adverse event rates. Disease-free survival was defined as the time interval between the date of IRT and the date of out-of-field progression or the date of last follow-up. Overall survival was defined as the time interval between the date of IRT and the date of death from any reason or the date of last follow-up. Cancer-specific survival was defined as the length of time from the date of IRT to the date of death from the disease. A summary table was created, including monocentric/multicentric study, sample size, median age, and endpoints. Statistical analysis was not performed due to scarcity of data.
Search strategy and selection of evidence
A systematic search in PubMed, Scopus, and Cochrane Library databases was performed to identify full-length articles evaluating the efficacy of IRT as boost after EBRT in patients with NPC. ClinicalTrials.gov was searched for ongoing or recently completed trials, and PROSPERO was searched for ongoing or recently completed systematic reviews.
Inclusion criteria were: 1) Randomized controlled trials (RCTs), prospective, retrospective, and cohort studies; 2) Utilization of IRT; 3) Patients receiving/not receiving EBRT; 4) Reported quantitative outcome data.
Studies were identified using the following medical subject headings (MeSH) and keywords: “nasopharyngeal cancer”, “rhinopharinx cancer”, “radiotherapy”, and “intensity-modulated radiotherapy”. The search was restricted to English language. The Medline search strategy was (“Brachytherapy” [Mesh] OR “Brachytherapy” [All fields]) AND (“nasopharyngeal Neoplasms” [Mesh] OR “nasopharyngeal Cancer” [All fields] AND (“rhinopharinx Neoplasms” [Mesh] OR “rhinopharinx Cancer” [All fields]). To avoid missing relevant studies, we chose strategy with high sensitivity but low specificity. We reviewed only published full-text clinical studies on patients with NPC carcinoma reporting comparison of two cohorts of subjects treated with EBRT alone and with EBRT plus IRT boost. Conference papers, survey, letters, editorials, book chapters, and reviews were excluded. Time frame between 2002 and 2022 as years of publications was considered. Two independent authors (VL, Rome; RM, Catania) screened citations in titles and abstracts to identify appropriate papers. Eligible citations were retrieved for full-text reviews. Uncertainties about inclusion in the review were considered by a multicenter and multidisciplinary expert team (LT, RI, VV, Rome; GK, Lübeck; CS, Catania; FB, Sassari; AGM, Bologna). Finally, a committee (AP, BF, PC, MM, Rome) performed an independent check and definitive approval of the review.
Quality of evidence evaluation
Certainty of evidence of all selected outcomes was performed according to GRADE approach considering study limitations, imprecision, indirectness, inconsistency, and publication bias. Certainty level starts at higher pre-specified level for randomized controlled trials, but levels of certainty can be downgraded if limitations in one of the above-mentioned domains are detected. Evidence can be classified as high, moderate, low, and very low level of certainty.
Benefit/harm balance and clinical recommendation
Based on the summary of evidence, the expert team expressed one of the following judgments about the benefit of risk ratio between intervention and comparison: favorable, uncertain/favorable, uncertain, uncertain/unfavorable, and unfavorable. The strength of recommendation can be considered as strong positive, conditional positive, uncertain, conditional negative, or strong negative.
Results
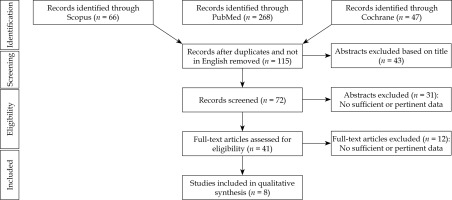
The literature search resulted in 381 articles after exclusion of conference papers, surveys, letters, editorials, book chapters, and reviews; 41 papers were assessed via full-text for eligibility. Of these, 32 articles were excluded for the following reasons: the abstract was not relevant, data was not relevant or incomplete, or did not follow the present review inclusion criteria. At the end of the process, 1,320 patients from 8 studies [12-19] were included in the systematic review. Figure 1 depicts PRISMA flow chart [20], and Table 1 summarizes the main characteristics of included studies. Six hundred and seventy patients received IRT and EBRT (IRT group), and 650 were treated with EBRT alone (no-IRT group). The mean patients’ age was 45 years (range, 9-83 years).
Table 1
Studies characteristics
| Authors [Ref.] | Period | Study | No. of patients | Treatment | LC | OS | DFS | CSS | Toxicity | Median FU (months) | Main results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chao [12] | 2002-2013 | Retro | 232 | IMRT: 68-72 Gy ± IRT: 3-11 Gy CHT: 176 pts. | 5-year IRT: 98.1% (T1) No IRT: 85.9% (T1) (p = 0.020) IRT: 100% (T2) No IRT: 91% (T2) (p = 0.184) IRT: 84.6% (T3) No IRT: 87.1% (T3) (p = 0.957) | Cranial nerve palsy IRT: 7.3% No IRT: 3.7% p = 0.240 Lhermitte’s sign IRT: 8.1% No IRT: 6.5% p = 0.644 | 63.1 (6-138) | In T1 and T2 disease IRT boost (p = 0.029) and chemotherapy (p = 0.047) influenced local control independently | |||
| Leung [13] | 1999-2003 | Retro | 287 | IMRT: 66 Gy ± IRT: 10-12 Gy | 5-year IRT: 95.8% No IRT: 88.3% (p = 0.020) | 5-year IRT: 89.2% No IRT: 74.8% (p = 0.0021) | 5-year IRT: 96% No IRT: 94.6% (p = 0.40) | 5-year IRT: 94.5% No IRT: 83.4% (p = 0.0058) | Cranial nerve palsy IRT: 4.3% No IRT: 8.5% Soft tissue ulcer IRT: 0.7% No IRT: 0.8% Lobe necrosis IRT: 0% No IRT: 2.5% | DMFS and PFS rates for IRT and no IRT groups were 95% and 83.2% (p = 0.0045), and 89.2% and 74.8% (p = 0.0021), respectively. 5-year major complication-free survival rate was 89.5% for IRT group and 85.6% for no IRT group (p = 0.23) | |
| Levendag [14] | Retro | 72 pts.: Neoadjuvant CHT + 70 Gy EBRT + IRT boost 73 pts.: 70 Gy EBRT + cCHT | 70 Gy EBRT ± 11 Gy IRT | 5-year T1-2 N+ IRT: 100% No IRT: 86% (p = 0.023) 5-year T3-4 N0+ IRT: 89% No IRT: 85% (p = 0.463) | 96 | In early stage (T1-2N+), IRT improved LC | |||||
| Ren [15] | 2004-2008 | Retro | 141 | 101 pts.: EBRT 68 Gy (43 pts. CHT) 40 pts.: EBRT 60 Gy + IRT 16 Gy (19 pts. CHT) | 5-year IRT: 97.5% No IRT: 80.2% (p = 0.012) | 5-year IRT: 97.5% No IRT: 91.1% (p = 0.231) | 5-year IRT: 92.5% No IRT: 73.3% (p = 0.014) | 5-year IRT: 97.5% No IRT: 93.1% (p = 0.31) | Xerostomia IRT: 8 pts. No IRT: 28 pts. Ulcer IRT: 7 pts. No IRT: 19 pts. | 3D HDR-IRT achieved excellent local control for stage T2b NPC patients as a result of improved target coverage and conformality of the radiation dose applied | |
| Taheri- Kadkhoda [16] | Retro | 8 | SIMT-IMRT PTV-T: 72.6 Gy PTV-TN: 66 Gy PTV-N: 52.8 Gy 3D-CRT + IRT PTV-T: 72 Gy (IRT 6 Gy/2 fr.) PTV-TN: 66 Gy PTV N: 46 Gy | IMRT: 98% 3D CRT + IRT: 95.8% (p = 0.016) | Mean doses to middle/external ears, parotid glands, and temporomandibular joints were significantly lower in IMRT plans | Primary tumor mean equivalent uniform dose (IMRT 67 Gy vs. IRT 63.7 Gy, p = 0.016) | |||||
| Rosenblatt [17] | 2004-2008 | Retro | 273 | 139 pts.: Neoadjuvant CHT + RT-CHT 70 Gy 135 pts: Neoadjuvant CHT + RT-CHT 70 Gy + IRT-LDR 11 Gy HDR 9 Gy/3 fr. | 3-year IRT: 54.4% No IRT: 60.5% (p = 0.647) | 3-year IRT: 63.3% No IRT: 62.9% (p = 0.742) | 3-year IRT: 52.6% No IRT: 59.8% (p = 0.496) | Skin G1-2/3-4 IRT: 130/5 No IRT: 137/2 (p = 0.277) Subcutaneous tissue G1-2/3-4 IRT: 132/3 No IRT: 136/3 (p = 1.000) Mucous membranes G1-2/3-4 IRT: 131/4 No IRT: 138/1 (p = 0.209) Salivary glands G1-2/3-4 IRT: 126/9 No IRT: 131/8 (p = 0.806) Brain G1-2/3-4 IRT: 135/0 No IRT: 138/1 (p = 1.000) Trismus G1-2/3-4 IRT: 123/12 No IRT: 124/15 (p = 0.687) | 29 | 3-year OS < 40 years: 71.5% > 40 years: 57.6% (p = 0.014) 3-years LC < 40 years: 66.8% > 40 years: 51.1% (p = 0.058) 3-year OS T3-4N+: 50.6% Other: 66.5% (p = 0.024) 3-year DFS T3-4N+: 45.3% Other: 59.3% (p = 0.018) 3-year LC T3-4N+: 46.2% Other: 60.6% (p = 0.016) Stage and CHT with cisplatin/ 5 FU were adverse prognostic factors for survival | |
| Ozyar [18] | 1993-1999 | Retro | 144 | 106 pts.: EBRT 66 Gy (55 pts. CHT) + IRT 12 Gy/3 fr. 38 pts.: EBRT alone (27 pts. CHT) | 3-year IRT: 86% No IRT: 94% (p = 0.23) | 3-year IRT: 67% No IRT: 80% (p = 0.07) | IRT: 39.7% nasal congestion, transient nasal obstruction, 3% nasal mucosal synechy, 1 pt. optic neuropathy No IRT: 5 pts. G3-4 (2 pts. developed optic neuropathy, 2 pts. developed brain necrosis, and 1 pt. developed optic neuropathy and brain necrosis) | 32 | 3-year CSS: N0 100%, N1 79%, N2 89%, N3 60% (p = 0.0004) 3-year DFS: N0 93%, N1 70%, N2 80%, N3 46% (p = 0.0004) 3-year DMFS: N0 100%, N1 83%, N2 79%, N3 52% (p < 0.0001) 3-year LC: N0 93%, N1 79%, N2 95%, N3 95% (p = 0.03) | ||
| Yan [19] | 2009-2013 | Retro | 81 | Previous EBRT 64-88 Gy (median, 70 Gy) ± CHT Group A 125I IRT (39 pts.) 120 Gy (range, 100-140 Gy) Group B EBRT IMRT (42 pts.) 60-70 Gy (1,8-2 Gy/fr.) | 3-year IRT: 23.1% No IRT: 16.7% (p ≤ 0.05) | 3-year IRT: 30.7% No IRT: 32.6% (p = 0.346) | IRT: 10 pts. GIII/IV No IRT: 28 pts. GIII/IV, 6 pts. dead (p ≤ 0.05) | 30 | IRT: LC 6-12-24-36 mts.: 82-71-41-23% LTPFS: 21 mts. OS: 25 mts. OS 1-2-3 years: 84.6-51.3-30.7% No IRT: LC 6-12-24-36 mts.: 78-66-35-16% LTPFS: 17 mts. OS: 24 mts. OS 1-2-3 years: 85.7-50-32.6% Quality of life > IRT (p < 0.001) |
[i] CHT – chemotherapy, cCHT – concomitant chemotherapy, CSS – cancer-specific survival, IRT – interventional radiotherapy, HDR – high-dose-rate, EBRT – external beam radiotherapy, 3D-CRT – 3-dimensional conformal radiotherapy, IMRT – intensity-modulated radiotherapy, SIMT – simultaneous integrated multitarget, LDR – low-dose-rate, n – number, N – lymph nodes, LC – local control, DMFS – distant metastasis-free survival, RT – radiotherapy, OS – overall survival, PFS – progression-free survival, LTPFS – local tumor progression-free survival, Retro – retrospective, fr. – fraction, Gy – Gray, G – grading, pts. – patients, mts. – months, FU – follow-up
EBRT total dose ranged between 60 and 80 Gy. Iridium-192 IRT boost dose ranged between 6 and 20 Gy (Table 2). Only one study [19] treated patients with recurrent NPC with iodine-125 IRT alone (total dose of 100-140 Gy). The use of platinum-based chemotherapy was common but not uniform [12, 14, 15, 17, 18]. Most of the patients underwent 3D-IRT [12-18], and the applicators were positioned under topical anesthesia with fiberoptic endoscopic guidance in two studies [13, 15] and under CT guidance in one study [19]. LC, DFS, CSS, and OS were reported in eight [12-19], four [13, 15, 17, 18], two [13, 15], and four studies [13, 15, 17, 19], respectively.
Table 2
Equivalent dose in 2 Gy fraction (EQD2)
| Authors [Ref.] | EBRT dose | IRT dose | EBRT + IRT EQD2 (Gy) |
|---|---|---|---|
| Chao [12] | 70 (range, 68-72) Gy in 35 fractions | 3 Gy × 2 fr. (EQD2: 6.5 Gy) | 76.5 |
| Leung [13] | 66 Gy in 33 fractions | 5 Gy × 2 fr. (EQD2: 12.5 Gy) 6 Gy × 2 fr. (EQD2: 16.0 Gy) | 78.5 82 |
| Levendag [14] | 70 Gy in 35 fractions | 3.6 Gy × 3 fr. (EQD2: 15.1 Gy) | 85.1 |
| Ren [15] | 60 Gy in 30 fractions | 4 Gy × 4 fr. (EQD2: 18.7 Gy) | 78.7 |
| Taheri-Kadkhoda [16] | 66 Gy in 33 fractions | 3 Gy × 2 fr. (EQD2: 6.5 Gy) | 72.5 |
| Rosenblatt [17] | 70 Gy in 35 fractions | 3 Gy × 3 fr. (EQD2: 9.7 Gy) | 79.7 |
| Ozyar [18] | 66 Gy in 33 fractions | 4 Gy × 3 fr. (EQD2: 14.0 Gy) | 80.0 |
All studies reported 5-year LC rates; the median 5-year LC rate was 98% (range, 95.8-100%) and 86% (range, 80.2-91%) in the IRT and the no-IRT group, respectively [12-19]. Six of 8 studies found [12-16, 19] that 5-year LC was significantly higher in patients who received IRT compared with those who did not receive IRT [12-16, 19]. In two studies, age < 40 years (3-year OS, p = 0.014; 3-year LC, p = 0.058) [12] and chemotherapy (p = 0.047) [12, 17] were associated with better local control and OS [12].
Four of 8 studies reported 5-year OS rates. The median 5-year OS rate was 93.3% (range, 89.2-97.5%) and 82.9% (range, 74.8-91.1%) for the IRT and the no-IRT group, respectively [13, 15, 17, 18]. In one study, a higher 5-year OS rate was reported in the IRT group than in the group without IRT [13], while no significant differences were recorded in the other studies. Four studies reported 5-year DFS rates, and the median value was 94.2% (range, 92.5-96%) and 83.9% (range, 73.3-94.6%) for the IRT group and the no-IRT group, respectively [13, 15, 17, 18]. Two of 4 studies reported higher 5-year DFS rates in the IRT group.
Two studies reported 5-year CSS rates; in one study, CSS rate was significantly better in the IRT group compared with the no-IRT group (94.5% and 83.4%, respectively; p < 0.01) [13].
Severe late toxicity rates (grade 3-4) were reported in seven studies [12, 13, 15-19]. The most frequently reported severe late side effects were mucosal ulcers, major salivary gland toxicity, neuropathy, and cerebral necrosis (Table 1). No study reported statistically significant differences in terms of severe toxicity between the two groups. Finally, one study reported that quality of life (QoL) in the IRT group was significantly better than in the no-IRT group over 12-month follow-up (p < 0.001) [19].
EtD (evidence-to-decision) framework
The results of this systematic review suggested that IRT following EBRT might improve oncological outcomes in patients with primary early stage NPC compared with EBRT alone. However, since seven out of eight included studies were retrospective in nature, IRT could not be proposed as standard treatment for patients with primary NPC.
Benefit/harm balance and final recommendation
Based on the selected studies, the quality of evidence evaluation presented low level of certainty. Therefore, the expert team voted for the benefit/harm benefit as uncertain. The final recommendation stated by the expert team was in patients with early stage NPC. IRT as a boost after EBRT may be preferred after extensive discussion with the patient and in the presence of an experienced multidisciplinary team. The strength of the recommendation was considered conditional positive.
Discussion
This systematic literature review showed that IRT as boost after EBRT improved outcomes compared with EBRT alone in studies including patients with T1-2 NPC [12, 15, 17], without prohibitive toxicity and better results in terms of QoL. However, the retrospective nature of most studies that implies heterogeneity in treatment protocols (IRT total dose, schedule, and isodose prescription) and selection bias, together with the small sample size of all studies and short follow-up, hamper the possibility to definitely determine the role of IRT in this patient population. Despite these limitations, it is worth noting that most studies (75.0%) reported improvement in LC with IRT after EBRT compared with EBRT alone [12-16, 19], and in half of the series, the group of patients who received IRT also had better long-term rates of DFS [15, 18], OS [13], and CSS [13].
These encouraging data in terms of effectiveness and safety of IRT, corroborates recommendations issued by the Head and Neck Working Group of GEC-ESTRO (Groupe Européen de Curiethérapie, European Society for Therapeutic Radiology and Oncology) in 2009 on the use of low-dose-rate (LDR), pulsed-dose-rate (PDR), and high-dose-rate (HDR) IRT in head and neck squamous cell cancers [21]. According to these recommendations, patients with nasopharyngeal squamous cell carcinoma can be treated with a HDR-IRT boost of 18 Gy in 6 fractions (T1) or 12 Gy in 4 fraction (T2-4), after a break of 1-2 weeks from the end of EBRT (60 Gy in 30 fractions) [21]. In 2016, the GEC-ESTRO-ACROP (Advisory Committee for Radiation Oncology Practice) group published an update of recommendations on the use of IRT in squamous cell tumors of the head and neck due to the advent of image-guided IRT and endoscopic guidance [22]. This document highlights excellent results of IRT in the form of intracavitary brachytherapy, particularly in early cases, in which the local tumor extension can be adequately encompassed within prescription isodose, and encourages to exploit the potentiality of modern IRT in larger tumors by using the new endoscopy-guided intracavitary/interstitial devices [22].
Despite these positive results, IRT is only occasionally considered as treatment option in patients with NPC. Two Italian surveys confirmed that although this procedure is available, only a few centers routinely consider its use for NPC treatment [24, 25]. Such under-usage of head and neck IRT in clinical practice is probably due to both the general lack of practitioners with adequate expertise and experience, and the availability of highly conformal EBRT techniques, such as intensity-modulated radiation therapy or volumetric modulated arc therapy, which are easier to implement than IRT. Consequently, anatomic site-related multidisciplinary tumor boards (MDT) are frequently lacking experts in interventional procedures [26].
The current study has several limitations to consider. The number of included studies was small, and all studies were non-randomized. This has implications on the results since non-randomized studies carry several inherent preferences. Non-randomized trials do not use concealed randomization; hence, the groups may not be comparable, leading to selection bias. In addition, the presence of other confounding factors, such as unadjusted background variables, detection, and recall bias due to selective reporting or the presentation of incomplete outcome data, may affected the results of the analysis. Due to the small number of patients, important sub-group analyses could not be performed, such as clinical-pathologic factors and Epstein-Barr status, which were missing in all studies. Furthermore, no research reported the overall treatment time. As showed by Sharma et al., the local recurrence of disease increases with increased treatment time, and it is significant when the overall treatment time is above 100 days where BED lost becomes more than 0.10 Gy a day [27]. Toxicity data were extrapolated from retrospective studies with different outcomes and some with a short follow-up. However, longer follow-up is unlikely to lead to the detection of further differences in late toxic effects between the two groups. The scarcity of studies on this topic also highlights the importance of increasing awareness about the potential role of IRT in ameliorating NPC patients’ prognosis and QoL. Additionally, a valid option could be to retrospectively analyze collected data in a standardized manner across different centers [28-30].
Conclusions
This systematic review suggests that IRT may complement the role of EBRT in T1-2 primary NPCs allowing for long-term disease and survival outcomes that are at least comparable, if not better, than EBRT alone, while possibly reducing side effects and ameliorating patients’ QoL. Further improvements of IRT results might derive from the introduction of the newest 3D-image-guided or endoscopy-guided techniques. Future studies are warranted to better define the potential role of this promising but rarely used technique.