Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) belongs to a group of serine proteases responsible for the activation of precursor proteins and their transformation into active forms. PCSK9 has a significant effect on the serum level of low density lipoprotein (LDL) modulating LDL receptor (LDL-R) degradation. The enzyme was discovered by Seidah et al. in 2003 [1]. The PCSK9 gene is located on the short arm of chromosome 1. Gain-of-function mutations are associated with hypercholesterolemia and an increased risk of ischaemic heart disease [2], whereas loss-of-function mutations cause a reduction in the serum LDL level [3] PSCK9 has been detected in numerous organs, such as intestines, lungs, kidneys and brain, but the largest reservoir of this protein is the liver [4–6].
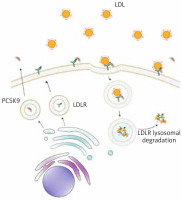
PCSK9 protein is synthesized as a proenzyme which undergoes autocatalysis in the endoplasmic reticulum. It consists of a prodomain, similar to the subtilisin catalytic domain and of the C-terminal segment rich in cysteine and histidine [7]. PCSK9 circulating in blood serum binds to a transmembrane LDL-R on the cell surface. After internalization of this complex into the hepatocyte, PCSK9 prevents recycling of the receptor and its return to the cell surface, promoting its lysosomal degradation [8]. This leads to a reduction in the LDL-R level on the hepatocyte surface and accumulation of circulating LDL [9] (Figure 1).
Figure 1
Role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in lipid metabolism. PCSK9 (brown) binds to the low-density lipoprotein receptor (LDL-R) (green), promoting its lysosomal degradation
Strong inducers of PCSK9 expression include sterol-regulatory element-binding protein 2 (SREBP2) activated by a low level of intracellular cholesterol, as well as hepatocyte nuclear factor 1α (HNF-1α) [10]. Serum PCSK9 level depends on i.a. oestrogens, growth hormone and thyroid-stimulating hormone concentrations, diet, and use of statins [11–15]. The PCSK9 concentration is also correlated with the body mass index (BMI), increased level of triglycerides, insulin and glucose, or the homoeostatic model assessment for insulin resistance (HOMA-IR) [16, 17].
Increased serum PCSK9 levels are positively linked with a higher risk of cardiovascular events, especially in male patients as well as in diabetic patients [18]. The most obvious mechanism relating atherosclerosis to PCSK9 is the PCSK9 involvement in the LDL receptor degradation. However, PCSK9 itself plays a role in the development of atherosclerosis, regardless of lipid changes. It has been shown that suppression of PCSK9 expression reduces oxidised LDL-induced synthesis by proinflammatory cytokine macrophages through the inhibition of nuclear factor-κB (NF-κB) activation and translocation, and inhibits endothelial apoptosis as a result of caspase 3 and 9 inhibition [18, 19].
An increased PCSK9 level is correlated with increased levels of white blood cells, C-reactive protein (CRP) and fibrinogen in patients with a coronary heart disease [20]. It has been shown that circulatory PCSK9 is correlated with the severity of liver steatosis in non-alcoholic fatty liver disease (NAFLD) [21]. The latest studies reveal an increased PCSK9 expression and release in other, aside to metabolic syndrome, chronic diseases, such as chronic kidney disease, hyperinsulinemia, hypothyroidism, and also in the course of inflammatory process, which is an important factor in the development of atherosclerosis [22–24]. In experimental studies on lipopolysaccharide-induced sepsis, PCSK9 overexpression was correlated with increased IL-6 output, and mice with PCSK9 gene knockdown revealed a reduced level of proinflammatory cytokines, e.g. IL-6, MCP-1, TNF-α, and MIP-2. Similar results were achieved when patients with septic shock and loss-of-function or gain-of-function mutations in the PCSK9 gene were compared [25, 26]. This confirms a direct proinflammatory effect of PCSK9. There has been a growing interest in mutual correlations between PSCK9 and inflammation in chronic and autoimmune diseases [25, 27].
The aim of this paper was to present the role of PCSK9 in the pathophysiology of skin disorders, specifically psoriasis and systemic lupus erythematosus, on the basis of the latest publications. The search was performed with regard to articles available in PubMed, published in 2015–2020. The keywords used were: PSCK9, psoriasis, lupus erythematosus, skin disorders, inhibitors PCSK9. The number of articles detected with particular keywords was 251. In the next stage 11 articles describing original research on a human or animal model that involved groups with psoriasis or lupus erythematosus or case reports concerning skin disorders and PCSK9 inhibitors were manually selected to be included in this narrative review.
PCSK9 and psoriasis
Numerous studies on psoriasis show a higher incidence, as compared with the general population, of associated diseases, lipid disorders, obesity, arterial hypertension, type 2 diabetes or liver function impairment [28–30]. An increased risk of cardiovascular events is especially dangerous. There is evidence that patients with psoriasis have a 5-year shorter lifespan, and the most common cause of death is myocardial infarction and thromboembolic incidents [31]. This mostly stems from the association of psoriasis with lipid metabolism abnormalities underlying the atherosclerosis initiation and progression. Psoriasis has been shown to be associated with higher levels of LDL and triglycerides, and lower HDL levels [32]. Moreover, oxidised LDL is present in psoriatic epidermis, but not in healthy skin from psoriatic patients [33]. A correlation between antibodies against these modified lipoproteins and the psoriasis severity has been observed [33]. The association of psoriasis with lipid metabolism disorders has genetic, environmental and immunological background; also, a significant role of an inflammatory process is emphasised. There are ongoing studies aiming to explain mutual correlations between these pathophysiological links. It has been observed that adipocytes triggered by inflammatory mediators (as in psoriasis) produce CRP [34]. This explains a correlation between an increased number of adipose cells and chronic skin inflammation. Moreover, hypolipemising agents (statins) used in the treatment of hypercholesterolemia improve the efficacy of psoriasis treatment [35].
A study conducted by Cao et al. has shown a correlation between PCSK9 and JAKs and the extracellular signal-regulated kinases (ERK) signalling pathway [36]. ERKs are increased in psoriatic skin lesions compared with nonlesional psoriatic skin, and ERKs activity normalizes with psoriasis clearance [36]. A study published in 2019 by Luan et al. gave evidence of PCSK9 overexpression in skin lesions in patients with psoriasis [37]. A quantitative analysis has shown that the level of mRNA, the PCSK9 expression is about 5 times higher in the psoriatic plaques that in the healthy skin [37]. PCSK9 expression was also studied in mice treated with imiquimod (IMQ), which induces psoriatic lesions. A higher PCSK9 expression was shown in the skin treated with IMQ, as compared with the control group [37]. These results show that treatment with IMQ induces PCSK9 expression together with psoriatic lesions. On the other hand, mice with PCSK9 gene knockdown have not revealed psoriatic lesions after IMQ treatment [37]. These findings suggest a significant PCSK9 involvement in the formation of psoriatic plaques.
In a recently published study, the serum PCSK9 level was examined in correlation with disease severity, inflammatory process, metabolic syndrome and effect of systemic therapy in psoriatic patients [38]. The study showed an increased PCSK9 level in patients with psoriasis, regardless of the disease severity [38]. Moreover, a reduced PCSK9 level was observed in patients treated with methotrexate (MTX) for 3 months, while treatment with acitretin resulted in further increase in the PCSK9 concentration [38]. A study reported by Garshick et al. suggests a significant correlation between circulating PCSK9 levels and early and advanced stages of atherosclerosis in psoriasis, regardless of the cholesterol level [39].
PCSK9 and systemic lupus erythematosus
In 1976, Urowitz et al. reported an increased risk of cardiovascular diseases in patients with systemic lupus erythematosus (SLE) [40]. It is estimated that the risk of their incidence is 2–10 times higher than in the general population, while the risk of myocardial infarction in premenopausal women increases by 50 times as compared with the healthy control group. However, the mechanisms underlying these observations have not been fully clarified yet [41]. They may only partially be explained by the presence of typical cardiovascular factors, e.g. disorders in blood lipids, which is confirmed by no reduction in the cardiovascular risk among SLE patients after implementing statin treatment [42–44]. Also, a significant role of inflammation in increasing the risk of atherosclerosis in SLE patients is emphasized [42].
Chenglong et al. assessed the serum PCSK9 level in a group of 90 SLE patients and 50 controls [45]. All the SLE patients had an on-going systemic treatment, some with more than one drug: all the patients were treated with hydroxychloroquine, 75 patients with glucocorticoids, 15 with azathioprine, 9 with cyclosporine A and 16 with mycophenolate mofetil. No differences between the control and the SLE group were revealed with respect to classic cardiovascular risk factors, such as age, sex, body mass index, serum levels of total cholesterol, LDL, HDL, triglycerides, uric acid and fibrinogen. In contrast, a statistically significantly higher serum PCSK9 level was observed in the SLE group as compared to the control group. The authors suggested that PCSK9 might be responsible for the increased risk of cardiovascular diseases in the SLE patients [45].
The study of Liu et al. assessed the serum PCSK9 level, thickness of intima/media complex and the presence of atherosclerotic plaques in jugular arteries, as well as the role of PCSK9 in differentiation of dendritic cells from peripheral blood monocytes [46]. The research was conducted on a group of 109 SLE patients and 91 controls. In contrast to the study by Chenglong et al., no increased serum PCSK9 level was observed in the SLE patients as compared to controls. Of note, however, was a difference in age of the enrolled patients (mean 33 years vs. 48 years in the Chenglong et al. study). For the SLE patients, a statistically significant correlation was observed between the serum PCSK9 level and severity of the disease, as assessed by SLAM (Systemic Lupus Activity Measure, p = 0.020) and SLE Disease Activity Index (p = 0.0178) [47]. Additionally, a mechanism partially explaining the effect of PCSK9 on the course of SLE was suggested. SLE patients reveal a higher concentration of oxidised low-density lipoproteins (oxLDL). Also, an increased sensitivity to oxLDL activity, as compared with healthy subjects, may be observed. OxLDL promote activation and maturation of dendritic cells (DC), which belong to a group of PCSK9-dependent antigen-presenting cells [46].
Novel therapeutic strategies for hypercholesterolemia and skin disorders
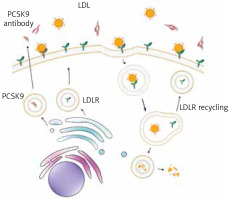
Monoclonal antibodies against PCSK9, evolocumab and alirocumab were approved by FDA in 2015 for the treatment, as monotherapy or combination therapy with statins, of patients with familial hypercholesterolemia (FH) or a high cardiovascular risk [48]. These agents prevent binding of PCSK9 to LDL-R by blocking LDL-R degradation, which results in a higher LDL receptor recycling and reduced serum LDL level, which in turn reduces the risk of cardiovascular events [47, 49] (Figure 2). Alirocumab was found to reduce the LDL concentration below 70 mg/dl in more patients with a high cardiovascular risk than ezetimibe [50]. Evolocumab alone significantly reduced the LDL level in FH patients, and additionally, when combined with statins, it statistically significantly reduced the LDL level and the risk of cardiovascular events, as well as the level of non-HDL cholesterol, apolipoprotein B, Lp(a) and triglycerides [51, 52].
Figure 2
Mechanism of action for proprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies. Monoclonal antibodies against PCSK9 (red) block binding of PCSK9 (brown) to the low-density lipoprotein receptor (LDL-R) (green) and prevent LDL-R degradation, which results in a higher LDL receptor recycling and reduced serum LDL level
What is interesting, a complete remission of xanthelasmas as a result of alirocumab treatment in a 50-year-old patient with a heterozygous form of familial hypercholesterolemia was reported [53]. Earlier, the patient had been treated with atorvastatin for more than 20 years, and with ezetimibe for 5 years before the introduction of alirocumab [54]. It has already been mentioned that the knockout of PCSK9 expression probably inhibits the proliferation and inflammation of keratinocytes. PCSK9 suppression has been suggested to inhibit the hyperproliferation of keratinocytes by inducing apoptosis [38]. A hypothesis can be made that PCSK9 could be a treatment target to help improve psoriasis, however, to the best of our knowledge, no data exist on this topic. In contrast, a case of psoriasis reactivation after 30-year remission period in a 76-year-old patient with heterozygous FH, after 6 months of treatment with evolocumab (140 mg every 14 days) was reported [54]. Kanda and Okajima described the occurrence of atopic dermatitis (AD) in a 43-year-old patient with heterozygous FH receiving evolocumab [55]. Other reports refer to erythematous, well-defined lesions on the dorsal part of the hands a few days after the administration of the first evolocumab dose in a 23-year-old female with homozygous FH [56]. Administration site reactions, occurring on average in 2.1% of patients using evolocumab, are the main type of skin adverse reactions [56]. The most common adverse reaction of alirocumab and evolocumab was myalgia [56].
In summary, currently available monoclonal antibodies against PCSK9 may cause skin adverse reactions, which, in many cases, are mild and do not constitute a contraindication to treatment continuation.
Conclusions
Certain cardiac and skin diseases have a common pathophysiological pathway. This may explain the situation that psoriasis and SLE are associated with an increased risk of cardiovascular events. Although studies on the animal model demonstrated some promising data, showing PCSK9 gene knockdown may prevent the development of IMQ-induced psoriatic plaques, a limited number of reports on the PCSK9 role in mentioned diseases, small study groups, no conclusive data on the molecular mechanisms of the PCSK9 effect on the disease necessitate further research.








