A global health emergency was declared by the World Health Organisation in January 2020 following the outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the causa-tive agent of coronavirus disease 2019 (COVID-19) infection. As of April 2021, there have been over 140 million reported cases and over three million COVID-19 associated deaths worldwide [1]. Whilst the clinical presentation of COVID-19 infection is hugely varied, in critically ill patients concomitant abnormalities of coagulation have been seen with an unusually high incidence of arterial and venous thromboembolic complications [2–4]. Evidence of hypercoagulability, rather than consumptive coagulopathy, in conjunction with hypofibrinolysis appear to contribute to morbidity and mortality [5]. Both factors have been demonstrated at both the macrovascular and microvascular level with high incidence of pulmonary embolism and other venous thromboembolism [6], in addition to post-mortem findings of microangiopathy and micro-thrombosis in the lungs and other organs [7, 8].
As a consequence, the International Society on Thrombosis and Haemostasis (ISTH) developed guidelines in which they recommended that all patients hospitalised with COVID-19 infection were considered for thromboprophylaxis with either unfractionated heparin (UFH) or low molecular weight heparin (LMWH) [9]. The ongoing propensity for thromboembolic complications, often despite prophylactic anticoagulation however, has led to the need to develop a greater understanding of the mechanisms driving hypercoagulability associated with COVID-19 infection in order to inform the optimum thromboprophylaxis strategy in these individuals [10].
Standard coagulation tests including prothrombin time (PT), international normalized ratio (INR), thrombin time (TT) and activated partial thromboplastin time (aPTT) are limited in their ability to accurately reflect the severity of the prothrombotic phenotype observed in severe COVID-19 infections [11]. Here, the role of rotational thromboelastometry (ROTEM) as a near bedside test allowing a more comprehensive assessment of haemostatic function in the context of COVID-19 infection will be considered. In this narrative review we aim to consider, firstly, whether ROTEM analysis allows the prediction of thromboembolic complication, and secondly, whether it able to identify a specific subgroup of patients who would benefit from an individualised approach to anticoagulation based upon these results.
SEARCH STRATEGY
An electronic search using the PubMed database was performed with articles published between January 2020 and April 2021 considered for review. The search was performed using the keywords “COVID-19” OR “SARS-CoV-2” AND “Rotational Thromboelastometry”, which yielded twenty-two results. Twelve original articles were included for analysis and two existing literature reviews [12, 13] were found to be relevant. From the reference lists of these two review articles, four further relevant original studies were extracted and included for analysis. From the original search criteria, publications were excluded if they included healthy subjects alone or a paediatric population. Furthermore, studies published in a language other than English, case reports and comment articles were excluded from the analysis.
ROTEM TESTS
ROTEM allows real-time evaluation of the change in viscoelastic properties of whole blood during clot initiation, formation, stabilisation and lysis [14]. Unlike conventional coagulation tests using plasma alone, ROTEM offers the advantage of providing information about platelet function, degree of fibrinolysis and existence of hypercoagulability.
A ROTEM analysis includes assays evaluating intrinsic (INTEM) and extrinsic coagulation (EXTEM) pathways. The INTEM assay uses phospholipids and ellagic acid to activate and assess coagulation through the intrinsic pathway while the EXTEM assay utilises tissue factor to assess the extrinsic pathway. In addition to these variables, ROTEM also evaluates the isolated role of fibrin formation and polymerisation in clot formation (FIBTEM), which is achieved through the addition of the platelet inhibitor cytochalasin D [15].
The following parameters are described (Figure 1):
FIGURE 1
Graphic representation of ROTEM variables. A – clotting time (CT) in seconds, B – clot formation time (CFT) in seconds, C – maximum clot firmness (MCF) in mm, D – maximum lysis (ML) as a % of MCF
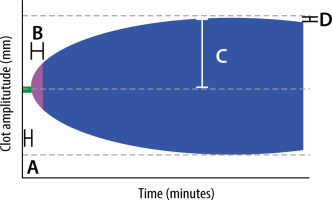
Clotting time (CT) – time elapsed in seconds from the start of measurement until a clot 2 mm in amplitude is formed. The amplitude recorded at 10, 20 and 30 min is referred to as A10, A20 and A30 respectively. The CT provides information about clot activation and initiation. The reference range for EXTEM CT is 42-74 seconds [16].
Clot formation time (CFT) – a measure in seconds of the propagation phase of whole blood clot formation from a clot amplitude of 2 mm to 20 mm. Reduced CFT is indicative of hypercoagulability. The reference range for EXTEM CFT is 46–148 seconds [16].
Maximum clot firmness (MCF) – the maximum amplitude, in millimetres, reached during the test which provides information about the final strength of the clot. The reference range for MCF is 49–71 mm (EXTEM) and 9–25 mm (FIBTEM) [16].
Maximum lysis (ML) – defined as the difference between MCF and the lowest clot amplitude after MCF, reflecting fibrinolytic activity and clot stability.
SUMMARY OF FINDINGS
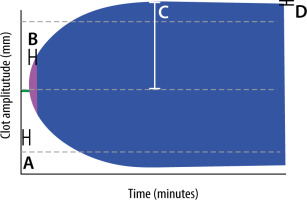
Presently, ROTEM tests in the context of COVID-19 infection have been utilised predominantly in a research setting to characterise the coagulation profile of these patients. We found sixteen original studies, all from high-income countries, describing the use of ROTEM in COVID-19 (Table 1). The summary of the findings displays homogeneity of coagulation abnormalities consistent with both hypercoagulability (reduced clot formation time and markedly increased maximum clot firmness) and hypofibrinolysis (reduced maximum lysis), with a typical ROTEM result depicted in Figure 2.
TABLE 1
Studies assessing coagulation parameters in COVID-19 patients using ROTEM
FIGURE 2
Graphic representation of ROTEM variables in COVID-19 patients. a – clotting time (CT) in seconds, B – reduced clot formation time (CFT) in seconds (compared to Figure 1), C – supra-normal maximum clot firmness (MCF) in mm. Dashed grey line represents normal MCF; d – reduced maximum lysis (ML) as a % of MCF (compared to Figure 1)
Pavoni et al. [17] retrospectively evaluated the ROTEM results of forty critically unwell patients with COVID-19 and demonstrated that these patients displayed a hypercoagulable state persisting over time despite treatment with appropriate thromboprophylaxis. A reduction in the CFT (INTEM and EXTEM) implied acceleration of the propagation phase of clot formation and increased MCF (INTEM, EXTEM and FIBTEM) and was consistent with a higher clot strength. The finding of increased MCF in ICU COVID-19 patients in comparison to non-COVID-19 ICU patients was corroborated by further research by Pavoni [18], Hoechter [19] and Boss [20]. The work of Boss and colleagues demonstrated the presence of more pronounced hypercoagulability in severe COVID-19 infection when compared with patients with severe sepsis with higher MCF (EXTEM and FIBTEM), higher fibrinogen and D-dimer levels and significantly reduced ML. Interestingly, the subgroup (albeit small) of patients with COVID-19 infection who developed thromboembolic complications showed no statistically significant differences in their ROTEM parameters in comparison to COVID-19 patients without thromboembolism [20]. Serial ROTEM results that were taken and analysed from thirty ICU COVID-19 patients on successive occasions between the day of admission to the ICU and day 14 in the work of Corrêa et al. [21] provide further support for the persistence of the hypercoagulable state over time. Similarly, Spiezia et al. [22] compared ROTEM profiles in twenty-two ICU COVID-19 patients with forty-four healthy, matched controls and found a comparable reduction in CFT (INTEM and EXTEM) and increased MCF in all assays. These ROTEM findings of hypercoagulability have been further corroborated by the work of van Veenendaal et al. [23], who analysed ROTEM variables in 47 ICU COVID-19 patients who displayed a reduced CFT (INTEM and EXTEM) and increased MCF. Interestingly, and contrary to expectation, COVID-19 patients with thromboembolic complications had an increased CFT (INTEM and EXTEM) and lower MCF (EXTEM) compared to patients without complications. The finding of a lower, albeit still supra-normal, MCF in COVID-19 ICU patients with venous thromboembolism was reiterated by the work of Roh et al. [24]. Almskog et al. [25] made an interesting observation in their study of ROTEM parameters in sixty hospital inpatients with COVID-19 infection. When comparing patients requiring a higher level of care to those with milder illness, they found that the markers of hypercoagulability (reduced CFT and increased MCF) were more pronounced. These findings are further supported by the work of Mitrovic et al. [26], who sub-categorised patients into moderate, severe and critical COVID-19 infection and demonstrated that hypercoagulable ROTEM patterns including reduced CT, increased MCF and reduced ML were more frequently observed with increasing disease severity. The only slightly contradictory findings come from a study by Blasi et al. [27], who demonstrated largely normal ROTEM parameters in twenty-three COVID-19 patients (ICU and general ward level care) when compared to reference values. They did, however, demonstrate an elevated MCF in some patients, and their in-depth data analysis suggested that low-therapeutic anticoagulant regimens appeared insufficient to downregulate the significant coagulation activation in COVID-19 patients. Van der Linden et al. [28] conducted a retrospective ROTEM analysis of two groups of ICU COVID-19 patients comparing the incidence of thromboembolic events in a standard thromboprophylaxis vs. an enhanced dosing regimen. In the first cohort with standard dosing LMWH, the MCF (INTEM, EXTEM and FIBTEM) was elevated above the upper normal reference limit in the majority of patients, indicating hypercoagulation. In the second cohort of enhanced dose anticoagulation, INTEM and EXTEM MCF were similarly elevated, whereas fibrinogen dependent (FIBTEM) MCF was significantly lower than in the first cohort and associated with a non-significant reduction in thromboembolic events.
Among twenty-one ICU COVID-19 patients, Creel-Bulos et al. [29] demonstrated that over 50% of patients met the criteria for fibrinolysis shutdown (EXTEM ML < 3.5%), which was not apparent on conventional coagulation testing. Of the cohort of patients who went on to develop thromboembolic complications, 89% were from the subgroup of patients with “fibrinolysis shutdown”. Likewise, the findings from Ibañez et al. [30] and Collett et al. [31] demonstrated supra-normal MCF and significantly reduced clot lysis in nineteen COVID-19 and six ICU COVID-19 patients respectively when compared to healthy control subjects. Lastly, Kruse et al. [32] used ROTEM analysis in a cohort of forty critically unwell COVID-19 patients. As with other studies, the MCF (INTEM, EXTEM and FIBTEM) was markedly elevated in the entire cohort while ML was reduced in INTEM and EXTEM. Under both conditions, ML was reduced and significantly lower in the group with thromboembolic complications. It was suggested by the authors that ROTEM analysis could serve as a potential tool for patient stratification according to their prothrombotic risk.
Of the sixteen aforementioned studies, all support the existence of marked hypercoagulability and reduction in clot lysis in the context of COVID-19 infection. Of particular interest is the possible association with disease severity and degree of hypercoagulability and hypofibrinolysis as a possible tool for risk stratification and the potential modulation of fibrinogen dependent MCF with enhanced anticoagulation strategies.
DISCUSSION
Virus-associated hypercoagulation is not a novel phenomenon and has been well documented in both severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV) infections [33]. With regard to COVID-19 infections, however, venous thromboembolic complications occur at twice the rate as seen with influenza infections, implicating unique pathophysiological factors beyond that of ‘sepsis-induced coagulopathy’ alone [2]. In general terms, the pathophysiology of hypercoagulability associated with COVID-19 includes the complex interaction between inflammatory and immune-mediated coagulation system activation resulting in elevated levels of fibrin-degradation products (D-dimer), fibrinogen and factor VIII levels, increased thrombin-antithrombin complexes and increased von Willebrand factor [34].
Our review expands on the previous knowledge on the topic, by summarising the results of the ROTEM studies in COVID-19 to date. A previous review by Tsantes et al. [12] concentrated on both ROTEM and other viscoelastic methods such as thromboelastography (TEG) and focused on the predictive ability of these techniques for patient outcomes. Compared to them we were able to include twice as many studies in our review with information on more than 500 patients compared to their sample size of around 180. Although many of the findings of the later studies summarised in our work have been confirmatory, it further strengthens our understanding on the utility of ROTEM in COVID-19.
Whilst the studies referenced in Table 1 do allude to a consistency and degree of reproducibility of coagulation abnormalities, it is worth noting several pertinent limitations with regard to the use of thromboelastometric testing in this context and within the studies themselves. Firstly, ROTEM is validated in the context of determining the cause of bleeding rather than in its ability to predict thrombotic events. As such, it could be argued that the use of ROTEM to identify subgroups of critically ill COVID-19 patients at most risk of thromboembolic events is beyond the realm for which it was originally designed. Secondly, the influence of the endothelium as an important co-factor of coagulation cannot be considered in ROTEM analysis. Lastly, Hardy et al. [35] recently published an article highlighting key intrinsic limitations of ROTEM in studies investigating haemostasis in COVID-19. They pointed out that ROTEM requires the use of unphysiologically elevated concentrations of EXTEM reagents to initiate clot formation and that fibrinolysis is initiated by endogenous, uninhibited plasminogen activators. These are often so low in concentration that fibrinolysis is negligible, which may, in part, explain the impaired maximum lysis parameters.
Owing to the relatively small sample sizes analysed in the studies, comparison and extrapolation of findings must be done with caution. Presently, there is no universal definition of hypercoagulability from analysis of ROTEM results. Between studies, criteria determining hypercoagulability were made by comparison to a number of different parameters including: established reference ranges; healthy matched and unmatched subjects; and different patient groups (non-COVID ARDS and surgical patients, for example). Moreover, there was considerable inter-study variability with anticoagulation in terms of the pharmacological agent (LMWH vs. UFH) used, the duration of anticoagulation treatment prior to ROTEM testing and dosing (standard vs. enhanced regime) of anticoagulation. Investigation regarding thromboembolic complications was poorly standardised across studies, with some relying on clinician discretion alone while others attempted to protocolise testing with serial lower-limb ultrasound scans. Finally, there was wide variability in terms of the timing of viscoelastic measurements during hospital admission, which will inevitability produce ROTEM results reflecting different stages of the disease course. With the changing haemostatic profile of patients critically ill with COVID-19 infection, the lack of standardisation makes assessment of the true degree of hypercoagulability and hypofibrinolysis challenging. To truly compare patient groups and assess therapeutic interventions, standardised ROTEM reference values are essential. It seems likely that thromboelastometric testing may provide a more physiologically representative insight into the coagulopathy associated with COVID-19. However, larger, multi-centre studies are needed to allow risk stratification of those at highest risk of developing thromboembolic complications. It is possible that ROTEM testing together with other more conventional assessment of hypercoagulation could reveal different clotting phenotypes, similarly as it has been described for hypo- and hyperinflammation in COVID-19 ARDS.
Clinical trials are currently underway investigating the utility of enhanced anticoagulation and fibrinolytic treatment approaches for COVID-19 patients. Recently, enrolment of patients requiring critical care in the three ongoing multiplatform, international trials addressing enhanced anticoagulation in COVID-19 patients (REMAP-CAP; NCT02735707, ACTIV-4; NCT04505774 and ATTACC; NCT04372589) were paused (as of December 21, 2020) due to an interim pooled analysis demonstrating futility of therapeutic-intensity anticoagulation in reducing the need for organ support over the first 21 days compared with standard-intensity prophylaxis in this patient subgroup. With this in mind, it appears that enhanced anticoagulation fails to target the relevant substrate(s) responsible for the high thromboembolic burden in critically unwell COVID-19 patients. A number of case reports investigating off-label administration of fibrinolytics (tissue plasminogen activator) in COVID-19 associated ARDS prompted the randomised, controlled phase IIa clinical trial (NCT 04357730) “STudy of Alteplase for Respiratory failure in SARS-Cov2/COVID-19”, which aims to test systemic administration of fibrinolytic therapy with tissue plasminogen activator (tPA; alteplase) versus standard of care for patients infected with COVID-19 resulting in severe respiratory failure. The results are anticipated in the near future and the secondary outcome measure of in-hospital coagulation-related events may provide insight into tPA as a potential therapeutic option in instances of “fibrinolytic shutdown” in patients who may benefit from this targeted therapeutic approach.
CONCLUSIONS
Severity of COVID-19 illness is associated with the degree of coagulation system activation with a high incidence of thromboembolic complications contributing to morbidity and mortality. The complex coagulopathy of this disease process appears to be over-simplified by conventional coagulation tests, which fail to allow the simultaneous assessment of both the coagulation and fibrinolytic components. Use of a global haemostasis assay such as ROTEM in COVID-19 patients, whilst not the perfect substitute for in vivo coagulation, has demonstrated increased maximum clot firmness (consistent with hyper-coagulability) and reduced maximum lysis (consistent with “fibrinolytic shutdown”). Precisely how these coagulation abnormalities can be modified by optimum, individualised medical interventions to improve clinical outcome, however, remains unclear. It is hoped that the upcoming publication of results from several ongoing clinical trials may shed some light on this complex matter.




