Purpose
Uterine serous carcinoma (USC) is an aggressive histologic subtype of endometrial cancer, similar to serous ovarian carcinoma. Although USC represents less than 10% of all endometrial cancers, it accounts for more than 50% of relapses and deaths attributed to endometrial carcinoma [1,2,3], with an estimated 5-year overall survival of 18-27% of patients with disease outside the uterus [4,5]. Also, in cases of disease confined to the corpus, the rate of relapse is high (31-80%), particularly in patients who are not surgically staged [6,7]. Some studies reported a 5-year survival rates of 15-30% of patients with clinical stage I USC [8,9] who underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH/BSO). Since the understanding of the importance of surgical staging in high-risk histologies has evolved, survival has improved. In the 25th Annual Report of FIGO, the 5-year survival rates for surgical stage I USC was 77% of patients who had not received any adjuvant therapy. The addition of adjuvant radiation therapy improved 5-year absolute survival for USC by approximately 8% [10]. However, the lack of prospective randomized studies does not allow to support optimal adjuvant treatment after surgery. Most of the available data are based on small, retrospective single- and multi-institutional studies [11,12]. Indeed, following the National Comprehensive Cancer Network (NCCN) guidelines, systemic therapy plus vaginal brachytherapy (VBT) or VBT in selected cases of non-invasive disease, represent valid options for stage IA serous carcinoma. While, for stage IB, systemic therapy plus or minus external beam radiotherapy (EBRT), and plus or minus VBT is recommended [13].
Despite these limitations, because of its aggressive behavior and pattern of recurrence, the treatment of USC is multimodal and it includes surgery, chemotherapy, and radiotherapy (RT). Adjuvant systemic platinum-based chemotherapy and radiotherapy after surgery showed an improved disease-free survival and overall survival with a reduction of recurrence rates [14,15,16,17,18]. In particular, VBT, in combination with chemotherapy, may play a very important role in patients with early USC because of its potential to provide an excellent dose distribution, shorter treatment duration, and preservation of the organ at risk.
The aim of this review was to examine efficacy of VBT after surgery and chemotherapy in stage I USC, in terms of disease-free survival (DFS), local control (LC), cancer specific survival (CSS), overall survival (OS), and safety.
Material and methods
A systematic research using PubMed, Scopus, and Cochrane library was performed in order to identify full articles evaluating the efficacy of VBT in patients with stage I uterine serous carcinoma. A search in ClinicalTrials.gov was conducted in order to detect ongoing or recently completed trials, and in PROSPERO for searching ongoing or recently completed systematic reviews. The studies were identified using the following medical subject headings (MeSH) and keywords including: “endometrial neoplasms”, “brachytherapy”, and “endovaginal radiotherapy”. The search was restricted to English language. The Medline search strategy was: (“Brachytherapy” [Mesh] OR “Brachytherapy” [All fields]) AND (“Endometrial Neoplasms” [Mesh] OR “Endometrial Cancer” [All fields]). To avoid missing relevant studies, we chose this strategy with high sensitivity, but low specificity.
We analyzed clinical studies only as full text of patients with stage I uterine serous carcinoma treated with VBT alone after surgical staging and adjuvant chemotherapy. Conference papers, surveys, letters, editorials, book chapters, and reviews were excluded. Patients who underwent previous treatments were excluded. Time frame from 1990 till 2018 as years of publication was considered.
Four independent authors expert in endometrial cancer regarding interventional radiotherapy and gynecological endometrial cancer screened citations in titles and abstracts in order to identify appropriate papers. Eligible citations were retrieved for full-text review. Uncertainties about their inclusion in the review were considered by a multicenter expert team.
The primary outcome was the DFS after VBT during follow-up. Secondary outcome included: LC, OS, CSS, and adverse event rates.
A summary table was created including sample size, median age, DFS, LC, toxicity, OS, and CSS.
Results
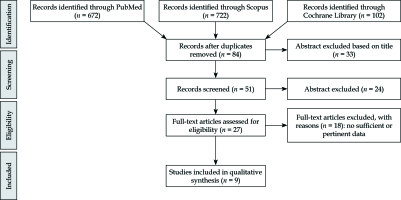
The literature search resulted in 672 articles. After exclusion by title and abstract, and after elimination of conference papers, surveys, letters, editorials, book chapters, reviews, and of non-English language, 27 papers were assessed via full text for eligibility. Of these, 17 articles were excluded due to insufficient data, leaving 9 studies assessing the clinical efficacy of VBT in DFS (Figure 1).
All studies were retrospective [19,20,21,22,23,24,25,26,27]. In accordance with the selection criteria, only data from the VBT treatment arms were extracted and considered for the analysis.
Our review identified 364 patients (with an average age of 67 years) with endometrial serous cancer, 331 with FIGO stage IA disease and 33 with FIGO stage IB.
All patients had undergone TAH/BSO. Pelvic lymph nodes dissection ranged from 80% to 100%, while para-aortic lymphadenectomy ranged from 35% to 100%. Peritoneal cytology ranged from 53% to 100% and omental sampling ranged from 57% to 100%. The presence of positive lymphovascular invasion (LVI) was reported in 46 patients out of 364 patients analyzed.
Adjuvant chemotherapy was administered with intervals of at least one week between chemotherapy and VBT. Chemotherapy consisted of platinum/taxane doublets.
Adjuvant VBT was delivered to the proximal two-thirds of the vagina and prescribed at an average dose of 21 Gy (range, 12-37.5 Gy) in 3 fractions at a depth of 0.5-0.7 cm [21,22] or at vaginal surface of the upper half of vagina [19,24,27].
The studies reported vaginal cuff, pelvic, and distant relapse in 1.6%, 7.2%, and 13.5% of patients, respectively.
The average LC was 97.5% (range, 91-100%), DFS was 88% (range, 82-94%), OS was 93% (range, 72-100%), CSS was 89.4% (range, 84.8-94%), and G3-G4 toxicity was 0%. Table 1 presents the characteristics of the included studies.
Table 1
Characteristics of the included studies
| Author | Period | Study | Size, n | Median age (years) | IRT (Gy) | CT | FU | DFS | LC | TOX G3-G4 | OS | CSS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tétreault-Laflamme et al. [19] | 2006-12 | Obser | IRT, 32 IRT + EBRT, 25 | 62 (47-83) 65 (52-79) | 31.05/3 fr 21/3 fr | Yes | 35 months | 3-y I, 88% 3-y I, 84% | 3-y I, 97% 3-y I, 100% | 0% | 3-y I, 100% 3-y I, 94% | |
| DuBeshter et al. [20] | 1995-01 | Obser | Stage IA, 10 Stage IB, 9 | 67 (27-96) | 21/3 fr | Yes | 5-y IA, 100% 5-y IB, 100% | n.a. | ||||
| Desai et al. [21] | 1996-10 | Obser | Stage IA, 56 Stage IB, 10 | 67 (27-88) | 21/3 fr | Yes | 62 months | 5-y IA, 91% 5-y IB-II, 78.5% | 5-y IA, 96.2% 5-y IB-II, 87.7% | n.a. | 5-y IA, 94.3% 5-y IB-II, 80% | |
| Kiess et al. [22] | 2000-09 | Obser | Stage IA, 30 Stage IB, 4 | 67 (51-80) | 21/3 fr | Yes | 58 months | 5-y I, 88% 5-y II, 71% | 100% | 8% | 5-y I, 93% 5-y II, 71% | |
| Mahdi et al. [23] | 2000-12 | Obser | Stage IA, 27 | 66 (49-90) | 21/3 fr | Yes | 47.7 months | 5-y IA, 88% | 5-y IA, 97.4% | n.a. | 5-y IA, 93.3% | |
| Qu et al. [24] | 2004-15 | Obser | Stage IA, 58 | 67.4 ±8.6 | 21/3 fr | Yes | 32 months | 5-y, 60.93% | 5-y, 82.99% | n.a | 5-y, 61.96% | 5-y, 79.99% |
| Robbins et al. [25] | 1998-09 | Obser | IRT, 33 IRT + EBRT, 23 | 64 (59-83) 69 (59-81) | 37.5 | Yes | 54 months | 5-y I, 85.6% 5-y I-II, 67.3% | 5-y I, 90.6% 5-y I-II, 71.8% | n.a. | 5-y IA, 71.9% | 5-y I, 84.8% 5-y I-II, 78.9% |
| Barney et al. [26] | 1998-11 | Obser | Stage IA, 74 | 66 (44-86) | 21/3 fr | Yes | 36 months | 5-y I, 92% | 5-y I, 100% | n.a. | 5-y I, 85% | |
| Donovan et al. [27] | 2000-14 | Obser | Stage IA, 21 | 68 (45-90) | 31.5/3 fr | Yes | 38 months | 5-y, 86% | 5-y, 96% | n.a | 5-y, 95% |
Discussion
The present review showed that IRT alone may be an adequate RT technique in women with stage I USC, who underwent appropriate surgical staging and received adjuvant chemotherapy.
Uterine serous carcinoma patients have a high-risk to develop local and distant relapse [28], therefore it is essential to ensure an adequate treatment even if patients with an early stage disease. While prospective studies have demonstrated that the delivery of chemotherapy in combination with external beam radiation in an advanced stage disease may increase survival benefit and decreases local recurrences [29,30,31,32,33], the role of radiation in early stage USC has been more difficult to demonstrate.
Owing to high-rate of distant recurrences, many studies recommended a more systemic approach to therapy for USC [34]. Treatment of women with uterine-confined USC with adjuvant chemotherapy is controversial due to lack of prospective randomized trials. However, in patients with stage I/II disease who were treated with observation only, a systemic adjuvant therapy is considered necessary on the basis of several retrospective studies. Only women who have undergone appropriate surgical staging and have no residual disease in the uterus at the time of surgery may not need further therapy [35,36,37]. The decision whether and how to treat women with stage I USC should be undertaken with careful consideration of the risks of relapse as well as of the treatment for each patient.
Given the rarity of this disease, all studies are retrospective and involve small numbers of patients, different histologies and stages, variable treatment regimens, and few events. This generates difficulties in interpreting the effectiveness of treatment regimens. The challenge in determining appropriate adjuvant treatment in stage I USC is, also, balancing a reduction in recurrence with treatment-related toxicity and complications. Indeed, following the NCCN guidelines, observations, chemotherapy, radiotherapy, or combination treatment are all valid therapies for patients with stage I USC [13]. Many studies have reported significant toxicity rates in stage IA patients treated with chemotherapy and pelvic EBRT, with a DFS remaining at around 85% [38,39,40]. While some centers have adopted VBT as the local therapy in this setting [23,41,42], many centers continue to use pelvic EBRT as a standard treatment [43,44]. In well selected stage I USC, VBT may be particularly useful in reducing local recurrences and toxicity. An adequate surgical staging is fundamental in guiding adjuvant therapy and defining patients who may undergo VBT. Many studies showed that the adequate staging is a factor associated with an improvement of DFS and OS [23,35,44]. There is general agreement that complete surgical staging in this high-risk population should consist of peritoneal cytology, omentectomy, and pelvic and para-aortic lymph node dissection; however, the specific nodal counts required (selective sampling vs. complete dissection), and the extent of omental and peritoneal evaluation remain controversial and institution-dependent. Three retrospective studies compared efficacy between EBRT and VBT in patients with stage I USC [19,25,45,46]. While Modh et al., in patients treated with VBT, reported longer 5-year survival rates than those treated with EBRT (VBT: 84% vs. EBRT: 75%; p < 0.001) [45], the other two studies showed no benefit from adding pelvic EBRT to VBT in terms of OS and LC [19,25]. These last results were confirmed by the Gynecologic Oncology Group 249 trial that showed no difference in OS or DFS between patients who received pelvic EBRT or VBT with three cycles of chemotherapy [46]. In the PORTEC-3 trial, women with serous or clear-cell cancers had at least as much improvement in failure-free survival with the addition of chemotherapy as women with endometrioid endometrial cancer. When comparing serous cancers with other histological types, worse OS and DFS were found for USC; patients with USC had a DFS benefit with chemoradiotherapy, but this benefit was not significant given the small number of USC and events [47]. Our results of DFS and OS are comparable with those reported in the GOG 249 and PORTEC-3 trial. Indeed, the present review suggests that the use of VBT in selected patients is associated with good outcomes in terms of LC (average 98.7%; range, 92.9-100%), DFS (average 88%; range, 85-95%), OS (average 93%; range, 90-94%), CSS 96.5% (range, 90-93%), and toxicity (range, 0-8%). The classic prognostic risk factors that guide treatment for endometrioid endometrial cancer, including depth of invasion, LVI, tumor size, and age, have not consistently been associated with prognosis for USC [43,44]. Stage IB [21], cervical stromal involvement [22], and lymphadenectomy [23] were an independent predictor of DFS and OS. The potential role of CA-125 as a tumor marker for USC remains unclear [43,44], but CA-125 may be valuable for monitoring disease [22]. Stage IA patients with residual uterine disease should receive concomitant VBT and platinum-based chemotherapy.
There are various fractionation schemes used clinically with no general consensus about the superiority of one regimen over the other. Traditionally, the doses for brachytherapy have been formulated to deliver approximately 60-65 Gy low-dose-rate (LDR) equivalent to the vaginal surface. Several institutions used different dose fractionation regimens, which have achieved acceptable outcomes based on their own published experience. There have not been single randomized trial comparing all these regimens. The most recent survey of vaginal brachytherapy practice [48] found that the most commonly used fractionation scheme is 7 Gy × 3 prescribed to 0.5 cm depth, followed by 6 Gy in 5 fractions prescribed to vaginal surface, and the last most common were 5.5 Gy × 4 and 5 Gy × 5 to 0.5 cm depth, and finally 7.5 Gy × 5 prescribed to vaginal surface. All these regimens appear effective based on institutional reports. Also, our review showed that the most common fractionation scheme was 7 Gy × 3 prescribed to 0.5 cm depth. Only 3 studies [19,25,27] used different schedules.
Regarding late toxicity, our review showed that the treatment is very well tolerated. The main side effects consisted of grade G1-G2, while G3-G4 late vaginal toxicities have been reported only in few cases (range, 0-8%) in line with the current data in literature [49,50].
Despite these positive results, VBT is not always considered as a treatment option in patients with stage I USC. Different Italian survey confirmed that despite this procedure is available, only few centers considered it for the treatment of USC [42,51]. Probably, the lack of experience, expertise, and treatment complexity do not allow the use of VBT in the clinical routine. VBT allows to deliver high doses of radiation to tumors, with minimal exposure of adjacent organs at risk. 3-dimensional computed tomography-based treatment planning offers all the advantages of a personalized treatment to achieve the optimum therapeutic index. Nevertheless, it is important that in no experienced centers, VBT can result in crucial side effects [52]. Considering the rarity of this disease, every case of stage I USC should be discussed with individual approach by an expert multidisciplinary team to provide more homogeneous treatment methods and improvement of clinical outcomes [53,54,55].
The possibility to identify a subgroup of patients with better survival prognosis could be used to offer VBT as a treatment, resulting in better quality of life. Several studies proposed a prognostic model, nomogram, or large-database to help identify the best strategy in individual patients [56,57,58,59].
Conclusions
Based on our review, we suggest that chemotherapy remains a critical component of treatment given the high rates of distant recurrence, while VBT appears sufficient to reduce local relapse without pelvic EBRT.
Larger, multicenter, randomized studies are required to determine the appropriate adjuvant therapy for patients with stage I USC, and to further characterize risk factors for recurrence and progression.