Purpose
Uveal melanoma, although a rare sub-class representing less than 5% of all melanoma diagnoses, is the most common primary intra-ocular malignancy in adults. The incidence has remained stable since the 1970s, with age adjusted incidence at 5.2 per million and 2,000 new cases per year in the United States [1-3].
Episcleral plaque brachytherapy is the most common treatment modality in the United States. A collaborative ocular melanoma study (COMS) trial found no significant difference in survival rates for medium-sized choroidal melanomas treated with enucleation vs. iodine-125 (125I) plaque brachytherapy, through 12 years of follow-up [4]. Another COMS trial reported a local recurrence rate of 10.3% at 5 years [5]. These findings established brachytherapy as an effective treatment option, with a major advantage over enucleation of globe preservation.
Local recurrence in uveal melanoma increases the risk of metastasis and disease-specific mortality [6, 7]. To this end, image-guided brachytherapy at the time of plaque placement has been investigated and cited to be important in helping to decrease local failure rates [8-11]. We report our single-institutional, three-year outcomes of episcleral plaque brachytherapy using eye physics 125I plaques for treatment of choroidal melanoma. This study expands upon a previous early outcomes report, now with a larger patient cohort and longer follow-up [11]. We demonstrated that detailed plaque planning and image-guided brachytherapy with intra-operative ultrasound at time of plaque placement is associated with increased local control.
Material and methods
The methodology utilized is detailed in the related previous early outcomes report and summarized below [11].
Patients
This study was approved by our facility’s institutional review board. Informed consent was obtained for experimentation with human subjects. Two-hundred twelve consecutive patients, with two-hundred fourteen uveal melanomas arising from the iris, ciliary body, and/or choroid, treated from January 2013 to December 2019, were included. Patients underwent comprehensive medical and ophthalmic evaluation, imaging to rule out distant disease, and fine needle aspiration biopsy to obtain genomic analysis, as previously described in the previous early outcomes report [11]. Baseline patients’ characteristics were recorded, including age, gender, tumor location and size, biopsy details, gene expression profiling (GEP) classification, and tumor stage. All patients were treated with eye physics iodine-125 episcleral plaque brachytherapy.
Plaque design and placement
Design of customized eye plaque treatment plan, plaque selection, seed activity calculations and verification, and planar reconstructions in plaque simulator software (Eye Physics, LLC, Los Alamitos, CA, USA) as well as dosimetric analysis via retinal dose-area histogram, were conducted [12, 13]. The methodology was as described in the previous early outcomes report [11]. Between 2013 and 2019, the following versions of plaque simulator software were employed: 576, 6.1.8, 6.4.7, and 6.6.2.
For most patients, modeling was performed for at least three plaque models, and the best design and plan was chosen based on three factors: 1) minimizing radiation dose to critical structures, 2) keeping maximum scleral dose under 400 Gy, 3) ease of placement/avoidance of the need to disinsert extra-ocular muscles, for patient comfort. The radioactive plaque was placed using coordinates determined from plaque simulator, and with utilization of intra-operative ultrasonography with/without additional intra-operative plaque localization via transillumination to verify appropriate plaque placement by a senior ophthalmic echographer. Plaques were adjusted intra-operatively whenever necessary. All surgeries were performed by one ocular oncologist, and all brachytherapy treatment plans were reviewed and approved by at least one radiation physicist, one radiation oncologist, one echographer, and one ocular oncologist.
Follow-up, endpoints, and statistical analysis
Follow-up examinations were conducted as described in the previous early outcomes report [11]. Data on local failure, distant metastases, adverse effects, and deaths were collected. Local failure was defined by intra-ocular tumor growth. Metastatic disease was defined by the first occurrence of metastatic disease by surveillance imaging. Local recurrence-free rate, distant metastasis-free rate, overall survival, and disease-specific survival were calculated using Kaplan-Meier method. Distant metastasis-free rate, overall survival, and disease-specific survival curves as stratified by GEP class were calculated by Kaplan-Meier method, and pair-wise log-rank test was used to compare survival curves. All statistics were calculated using SPSS Statistics software (IBM SPSS Statistics version 25, IBM Corporation, Chicago, IL, USA, 1989-2017).
Results
Patients, uveal melanoma, and treatment characteristics
Two hundred and twelve patients, and two hundred and fourteen tumors underwent 125I plaque brachytherapy treatment. One patient initially presented with two uveal melanomas, one in each eye, which were treated by two eye plaques at separate times. Another patient presented with two primary uveal melanomas at separate times within the left eye; both were nevi originally and observed, as they grew larger and developed clinical features of melanoma. Table 1 reports baseline characteristics of the patients and tumors in the study. The median age at presentation was 63 years (range, 19-91 years). There were 96 female (45.3%) and 116 male (54.7%) patients in the study. 111 (51.9%) tumors were located in the left eyes.
Table 1
Baseline characteristics of patients: tumor, treatment, and outcome
1 8th edition of the American Joint Committee on Cancer, 2 Collaborative ocular melanoma study, 3 Common terminology criteria for adverse events, * One patient presented with two UMs: one OS and one OD treated at separate times. One patient presented with two UMs at separate times within OS, treated at separate times, ** 180 patients had at least 12 months follow-up, for which outcomes are reported
211 of 214 lesions underwent biopsy via trans-vitreal or trans-scleral fine needle aspiration for GEP analysis. In all of these cases, ophthalmic pathologist reviewed the specimens in live time during surgery to confirm the presence of cells. Three patients opted not to undergo biopsy. According to GEP classification, 204 cases were successfully categorized: class 1A – 119 (55.6%), class 1B – 30 (14.0%), and class 2 – 55 (25.7%). Ten cases were not successfully classified: 6 cases with failure to amplify/threshold for analysis not achieved, and 4 patients declined the test.
Tumor height at diagnosis ranged from 1.1 mm to 3.3 mm for iris lesions, and from 1.4 mm to 14.5 mm for choroid/ciliary body lesions, with overall median height of 3.4 mm. Basal diameter ranged from 2.0 mm to 5.5 mm for iris lesions, and from 4.5 mm to 20.5 mm for choroid/ciliary body lesions, with overall median diameter of 11.5 mm. Uveal melanoma was staged according to the American Joint Committee on Cancer 8th edition, and they were: 81 cases stage I (37.9%), 60 IIA (28.0%), 47 IIB (22.0%), 18 IIIA (7.9%), 8 IIIB (3.7%), 0 IIIC, and 1 IV case (0.5%). COMS stage was as follows: 90 small (42.1%), 81 medium (37.9%), and 43 large (20.1%). Median dose at apex for tumor height > 5 mm was 85.0 Gy, and 120.6 Gy for tumor height ≤ 5 mm. The mean percentage of tumor volume receiving the prescription dose was 99.9% (SD ±0.6%), with a range of 94.9-100.0%. The mean percentage of tumor +2 mm margin volume receiving the prescription dose was 96.8% (SD ±2.8%), with a range of 85.0-100.0%. 15.4% of plaques were re-positioned after evaluation with intra-operative ultrasound guidance, to achieve satisfactory spatial coverage of the tumor.
Local recurrence, distant metastasis, and disease-specific survival
Outcomes data for 180 patients with at least 12 months follow-up were reported. In this subset of patients, the mean follow-up was 37.3 months and the median follow-up was 30.8 months (range, 12.2-96.3 months). 22 patients were lost to follow-up (defined as greater than one year since last assessment with exam). To date, no patients have experienced local recurrences.
Of patients with at least 12 months follow-up, 22 patients developed distant metastases after eye plaque treatment. Site of initial distant metastases was the liver in all 22 cases, with additionally lung (n = 2), bone (n = 3), or splenic (n = 1) sites of distant metastases. Of the 22 patients with distant metastatic disease, seventeen were GEP class 2, three were GEP class 1A, and two cases were without available classification (both with failure to amplify). Of the three patients with GEP class 1A who had developed metastatic disease, one patient had a discriminant value below the analysis confidence level and had extra-scleral extension on examination at presentation (a clinical risk factor for metastasis). The presumption in this case was that the tissue submitted for analysis was likely necrotic with poor quality RNA. Another patient case was PRAME-positive, a result, which may indicate an increase in metastatic risk for class 1 uveal melanomas.
Of patients with at least 12 months follow-up, there were 22 deaths, with 14 deaths attributable to metastatic uveal melanoma.
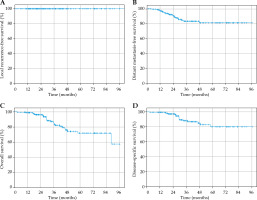
To minimize bias introduced by patients lost to follow-up, we also reported local recurrence-free survival, distant metastasis-free survival (DMFS), overall survival (OS), and disease-specific survival (DSS) (Figure 1) for all patients excluding those lost to follow-up (i.e., greater than one year since last assessment, n = 174). Local control remained at 100.0%.
Fig. 1
A) Kaplan-Meier curve for local recurrence-free survival. Local control remains at 100%. B) Kaplan-Meier curve for distant metastasis-free survival. Distant metastasis-free survival rate was 97.0%, 90.4%, and 81.1% at 1 year, 2 years, and 5 years, respectively. C) Kaplan-Meier curve for overall survival. Overall survival was 98.8%, 96.10%, and 71.9% at 1 year, 2 years, and 5 years, respectively. D) Kaplan-Meier curve for disease-specific survival. Disease-specific survival was 99.4%, 97.3%, and 80.2% at 1 year, 2 years, and 5 years, respectively
Distant metastasis-free survival, OS, and DSS curves stratified by GEP class are depicted in Figure 2. For comparison between GEP classes, cases in which GEP classification was unavailable (7 of 174 cases) were excluded. Pair-wise comparisons using log-rank test between GEP classes are reported in Table 2. A p-value of ≤ 0.05 was considered to be statistically significant. There was a statistically significant difference in DMFS, OS, and DSS between GEP class 1A and 2 as well as between GEP class 1B and 2. There was no significant difference between class 1A and 1B, with respect to DMFS, OS, and DSS.
Fig. 2
A) Kaplan-Meier curve for distant metastasis-free survival (DMFS) according to GEP classification. DMFS at 1 year, 2 years, and 5 years for class 1A: 98.9%, 97.5%, and 97.5%, respectively. DMFS at 1 year, 2 years, and 5 years for class 1B: 100.0%, 100.0%, and 100.0%, respectively. DMFS at 1 year, 2 years, and 5 years for class 2: 91.4%, 71.5%, and 42.2%, respectively. B) Kaplan-Meier curve for overall survival (OS) according to GEP classification. OS at 1 year, 2 years, and 5 years for class 1A: 98.9%, 96.2%, and 86.5%, respectively. OS at 1 year, 2 years, and 5 years for class 1B: 100.0%, 100.0%, and 90.9%, respectively. OS at 1 year, 2 years, and 5 years for class 2: 98.0%, 93.2%, and 37.9%, respectively. C) Kaplan-Meier curve for disease-specific survival (DSS) according to GEP classification. DSS at 1 year, 2 years, and 5 years for class 1A: 100.0%, 96.5%, and 96.5%, respectively. DSS at 1 year, 2 years, and 5 years for class 1B: 100.0%, 100.0%, and 100.0%, respectively. DSS at 1 year, 2 years, and 5 years for class 2: 98.0%, 93.2%, and 41.6%, respectively
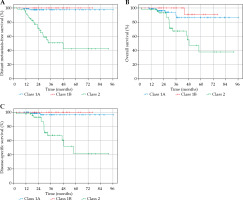
Table 2
Pair-wise comparisons using log-rank test between gene expression profiling (GEP) classes for distant metastasis-free survival, overall survival, and disease-specific survival
Adverse effects
Two patients had undergone enucleation for blind, painful eye, one at 18 months and the other at 30 months post-plaque treatment. 87 patients developed radiation-related toxicities. In particular, 85 patients (47.2%) developed radiation retinopathy during the study period. According to the common terminology criteria for adverse events version 5.0, 33 retinopathy cases were grade 1 (asymptomatic), 13 were grade 2 (symptomatic with moderate decrease in visual acuity), 18 were grade 3 (symptomatic with marked decrease in visual acuity), and 21 cases were grade 4 (best corrected visual acuity of 20/200 or worse in affected eye). There were no hospitalizations or deaths as a result of adverse effects. Of the 85 patients who developed radiation retinopathy, 45 patients were treated with anti-vascular endothelial growth factor therapy in the context of a clinical trial. The median time to radiation retinopathy was 24 months. Other radiation-related toxicities were rare, and included retinal vein occlusion, serous retinal detachment, vitreous hemorrhage, optic neuropathy, and hypotony maculopathy.
Discussion
Previously, we reported on early outcomes of uveal melanoma for 48 patients treated with episcleral 125I plaque brachytherapy, with 100% local control at a median follow-up of 21.6 months [11]. The present study not only included a larger patient population of 212 patients, but also reported local control rate of 100% at a longer mean follow-up of 37.3 months, with a low-rate of loss to follow-up (12.2%). Furthermore, this study involved a relatively high proportion of larger tumors (20.1% were classified large by COMS staging, vs. 6.3%). The excellent local control rate was likely attributable to utilization of 3 independent methods of plaque localization, such as 3D treatment planning, intra-operative transillumination, and intra-operative ultrasonography. These are described in detail in the early outcomes report [11].
Literature review for other studies utilizing intra-operative guidance for eye plaque placement
A limited number of reports in the literature describe the utilization of intra-operative ultrasound (IOU) guidance for eye plaque brachytherapy in uveal melanoma. Selected reports are summarized in Table 3.
Table 3
Literature review. Selected studies of eye plaque brachytherapy for uveal melanoma utilizing intra- operative ultrasound (IOUS) guidance for plaque placement
Tabandeh et al. examined 117 patients with only COMS medium-sized tumors that were treated with 125I episcleral plaque brachytherapy and intra-operative echo-graphic guidance. At a mean follow-up of 37 months, 1.7% of patients had local tumor recurrence and underwent enucleation [8].
Chang et al. reported on 150 patients treated with 125I eye plaque brachytherapy, of which intra-operative ultrasound guidance identified the need to reposition 36% of plaques. They reported 100% local control, with a mean follow-up of 21.5 months. They concluded that intra-operative ultrasonography reduces geographic errors, and may help to reduce local treatment failure in choroidal melanoma [9].
Badiyan et al. described outcomes of a cohort of 526 patients with posterior uveal melanoma treated with 125I plaque brachytherapy using intra-operative ultrasonography, with supplemental transpupillary thermotherapy (TTT) for selected patients at high-risk for local recurrence. At mean follow-up of 53.4 months, they reported an actuarial 3-year and 5-year local control rate of 97.3% and 95.4%, respectively. They concluded that detection of plaque tilt by ultrasonography at plaque removal allows supplemental TTT to be used in patients at potentially higher risk for local recurrence [14].
Bellerive et al. examined patterns of recurrence in a cohort of 374 patients with posterior uveal melanoma treated with 125I or ruthenium-106 (106Ru) eye plaque brachytherapy. 316 cases incorporated intra-operative ultrasound guidance. Mean follow-up was 47 months. The study noted that 33.3%, 71.4%, and 90.5% of recurrence occurred within 1, 2, and 5 years post-treatment, respectively. They concluded that local recurrences were uncommon at 5 years after plaque brachytherapy [15].
Aziz et al. reported on their cohort of 252 patients treated with either 125I or 106Ru eye plaque brachytherapy. After incorporation of intra-operative ultrasound guidance in 2007 for 198 patients, they reported that their local recurrence rate decreased from 9.3% to 1.5%, concluding that intra-operative confirmation of plaque placement reduces risk of early local recurrence [10].
Literature review for long-term outcomes for uveal melanoma treated with 125I eye plaque brachytherapy
Several reports in the literature report long-term outcomes for 125I eye plaque brachytherapy for uveal melanoma without utilization of intra-operative ultrasound guidance [16-30]. Selected reports are summarized in Table 4. Local control rates were reported in the range of 86.9-96% at 2-3 years, 80-98.3% at 5 years, and 93-94.4% at 8-10 years. This broad summarization of local control rates is limited by potential variation amongst different studies by plaque placement methodology, radiotherapy planning, follow-up at reporting, and use of actuarial estimates. We report a local control rate at 100% with intra-operative ultrasound guidance at the time of plaque placement, with a mean follow-up of 37.3 months.
Table 4
Literature review. Selected studies reporting long-term outcomes for uveal melanoma treated with 125I eye plaque brachytherapy
Literature review on other treatment modalities (106Ru, 103Pd, proton, and particle therapy)
A few studies have compared 125I to other isotopes in eye plaque brachytherapy for uveal melanoma with respect to outcomes and toxicities. Takiar et al. performed a comparative analysis of 125I and 106Ru, with actuarial 5-year local control at 83% for 125I, and at 97% for 106Ru. They concluded that both 125I and 106Ru eye plaque brachytherapy resulted in encouraging tumor control, and 106Ru offers benefits with reduced toxicity in patients treated for uveal melanomas ≤ 5 mm in apical height [31]. Leonard et al. reported on their institutional experience treating uveal melanoma with 125I, 106Ru, and cesium-131 (131Cs). In their extensive literature review, for which can be referenced, 5-year local control rate for 106Ru averaged over five selected studies was 91.5%, and local control rate for 103Pd averaged over two studies was 96.3% [32].
Proton and helium particle radiotherapy as modalities to treat uveal melanoma are also reported in the literature. Lin et al. examined a National Cancer Database in comparing proton beam radiotherapy vs. episcleral plaque brachytherapy, and found that 5-year local control rate for proton therapy ranged from 85% to 96%. They also concluded that proton beam radiotherapy reported inferior overall survival when compared to episcleral plaque brachytherapy, which was statistically significant [33]. Verma et al. sought to review outcomes of proton radiotherapy for uveal melanoma, drawn from 14 studies from 10 institutions. They found that 5-year local control rates exceeded 90%, which persisted at 10 and 15 years. They reported that the rate of retinopathy varied from 23% to 67%, and concluded that proton beam radiation can reduce ocular toxicities in patients with uveal melanoma [34]. Mishra et al. reported on a prospective trial comparing helium particle radiotherapy to plaque treatment. Local control for particle vs. plaque treatment was 100% vs. 84% at 5 years, and 98% vs. 79% at 12 years (p = 0.0006). They concluded that helium particle therapy resulted in significantly improved local control, eye preservation, and disease-free survival [35].
Radiation retinopathy rates
Radiation retinopathy incidence increases over time, and cumulative incidence at a specified time-point after eye plaque treatment was not reported in several studies. Our reported rate of radiation retinopathy of 47.2% is compared with values from the literature [16,18-21,23-25,29,31,36].
Limitations to this study include its’ retrospective and non-comparative schema. In addition, follow-up duration was limited, and a longer study period is needed to strengthen the durability of finding of the high local control rate. In addition, a number of patients were lost to follow-up due to living far away from a tertiary referral center, which could potentially under-report local failures.
Nevertheless, several strengths of this study must be highlighted. There are a limited number of studies reporting on intra-operative image guidance for 125I eye plaque placement. To date, only one study has reported a local control rate as high as ours [9]. Our study is the largest (212 patients, 180 patients with at least 12 months of follow-up) and with the longest follow-up that reports a very high local control rate. Intra-operative image guidance is likely to contribute to accurate plaque placement, as evidenced by adequate coverage metrics (mean V85 Gy of 96.8%) and image-guided repositioning utilized in almost 1 in 6 plaques. Other studies have reported utilizing intra-operative ultrasound to reposition plaques for uveal melanoma in 14-90% of cases, citing this technique as an important factor in minimizing treatment failure [8, 9, 37]. Additionally, a majority of lesions were biopsied (211) at time of plaque placement, and had good cytologic yield and genomics results (186, or 88% of biopsied lesions), a critical component of the modern approach to treatment. Another significant strength of this study is that the 100% local control rate was maintained for uveal melanomas spanning across different stages, sizes, and GEP classifications. 20.1% of the tumors were classified large by COMS staging, yet excellent local control was maintained; this contrasts with many published papers, which only included COMS medium-sized tumors. This can be attributable to several factors, including 3D planning, image guidance, and various choices of eye physics plaques that collectively provide a personalized plaque, based on size and shape of the tumors, location of critical structures, max sclera dose, and tumor coverage.
The GEP (gene expression profile) classification was developed to distinguish between uveal melanomas that have a low metastatic risk (class 1 tumors) vs. high metastatic risk (class 2 tumors) [38]. This classification has been validated as the strongest prognostic factor for metastasis, over TNM classification and the previous gold standard, chromosome 3 testing [39, 40]. The five-year risk of metastasis for class 1A, 1B, and 2 tumors, as cited by DecisionDx-UM test, is 2%, 21%, and 72%, respectively [41]. In comparison, in our patient cohort examining patients with follow-up within the last year, the five-year risk of metastasis for class 1A, 1B, and 2 tumors in this series was 2.5%, 0.0%, and 57.8%, respectively. It has been well-established for decades and from international multicenter studies that local recurrence in uveal melanoma significantly increases the risk of distant metastasis and disease-specific survival [6, 42]. Our study demonstrated a significant difference between GEP class 1 and class 2 lesions in DMFS, OS, and DSS, which suggested, as expected, that class 2 lesions are the drivers for worse outcomes in these metrics. Of note, our study showed that an excellent local control was maintained despite a relatively high proportion (25.7%) of GEP class 2 lesions consistent with the distribution of previously published large series. Furthermore, our study demonstrated a lower five-year risk of distant metastases for class 1B and class 2 lesions as compared to the established rates afore-mentioned. We postulate that the methodology utilized here may be able to eradicate locally aggressive disease prior to development of micro-metastases, particularly in smaller tumors or tumors in transition from a less aggressive genotype to a more aggressive one. Future work would include longer follow-up of this cohort as well as a larger multicenter prospective evaluation of the effect of local control on metastases in different genomic sub-groups.
Conclusions
We report 0% local failure rate and favorable distant metastasis rate in uveal melanoma, with just 12% of patients lost to follow-up, in tumors ranging from small to large size, and with varying stages and GEP classes, who were treated with 125I eye plaque brachytherapy using intra-operative ultrasound guidance, showing an acceptable rate and severity of radiation-related toxicities. While we are unable to definitively determine critical factors important for our excellent local control, the use of careful pre-operative planning and image-guided brachytherapy with intra-operative ultrasound at the time of plaque placement, may contributed to these outcomes. Longer follow-up is necessary to validate the reported local control rate.


