Purpose
The primary treatment for locally advanced carcinoma cervix is radical chemoradiation. Radiation comprises of external beam radiotherapy (EBRT) over a period of five weeks, followed by brachytherapy. Over the last decade, there have been several advancements in brachytherapy for carcinoma cervix. GEC-ESTRO guidelines for image-guided brachytherapy [1, 2] have been adapted in various centers with improved outcomes over conventional point A-based brachytherapy. Magnetic resonance imaging (MRI) is the gold standard for target delineation in image-guided brachytherapy. Trans-rectal ultrasound (TRUS) has excellent soft tissue resolution, and has been used extensively in cervical cancer diagnosis. Among all ultrasound modalities, parametrial infiltration is best depicted on TRUS. Therefore, TRUS imaging can be as good as MRI in experienced hands, and can serve as a cost effective alternative to MRI [3, 4].
The main techniques of performing brachytherapy in carcinoma cervix include intra-cavitary (IC) brachytherapy and interstitial (IS) brachytherapy. Intra-cavitary brachytherapy is done using a tandem and ovoid or a tandem and ring applicator for patients with good response post-EBRT. Interstitial brachytherapy is performed using perineal templates in selected patients, where it is not possible to achieve IC brachytherapy with significant residual disease post-EBRT, or in distorted anatomy [5]. In the past few years, various hybrid brachytherapy applicators have been developed in order to improve target coverage and minimize doses to organs at risk (OARs) [6, 7]. These applicators are a modification of the standard IC applicators and incorporate an interstitial component along with the IC component. TRUS is routinely used to guide needle insertion and treatment planning in carcinoma prostate [8], but information regarding its use in carcinoma cervix interstitial brachytherapy is limited. Recent data has shown that TRUS can aid in tandem insertion and provide real-time image guidance for needle insertion, and thus help in avoiding the injury to OARs [9, 10]. The aim of the current study was to report the feasibility of performing combined IC and IS brachytherapy in carcinoma cervix patients under trans-rectal ultrasound guidance.
Material and methods
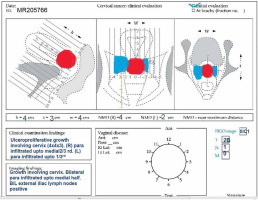
All patients who received chemoradiation with curative intent and planned for hybrid IC/IS brachytherapy were prospectively included for analysis. Patients with residual disease > 30 cc or target width > 4 cm, or asymmetric target at the time of brachytherapy were planned for hybrid brachytherapy. Patients, in whom the uterus could not be sounded, or when there was extensive residual disease involving mid and lower vagina or lateral parametrium, were excluded. An ethical and scientific review board approval was obtained prior to the study. A thorough clinical examination was performed at presentation and documented in the form of clinical diagrams (Figure 1). Diagnostic investigations included a biopsy, baseline blood investigations (complete blood picture, blood urea, and serum creatinine), chest X-ray, and MRI of abdomen and pelvis. A whole body PET-CT scan was performed in patients who agreed for the scan, but it was not compulsory. Response assessment post-EBRT was documented in clinical diagrams by performing clinical examination and MRI before brachytherapy.
EBRT and chemotherapy details
All patients received an external beam radiotherapy dose of 50 Gy in 25 fractions at 2 Gy per fraction, five days a week, for five weeks along with weekly chemotherapy (inj. cisplatin 40 mg/m2). 3D-CRT or image-guided radiotherapy (IGRT) as EBRT technique were used.
Brachytherapy procedure details
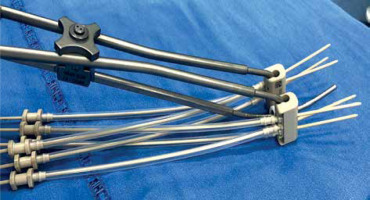
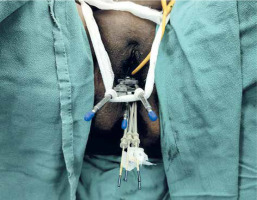
All patients were assessed for feasibility of brachytherapy in the last week of EBRT. Brachytherapy was performed within ten days of completion of EBRT using Fletcher style tandem and ovoid applicator with interstitial component (Varian, Figure 2). A detailed QA and commissioning were completed prior to the usage of this applicator. After administering of general anesthesia, the patient was placed in lithotomy position and the bladder was catheterized. Post-catheterization, the bladder was filled with 100 cc normal saline to facilitate sounding and tandem insertion in case of acutely anteverted uterus. All patients were administered single prophylactic dose of intravenous antibiotic just before the procedure, according to an institutional protocol. Examination under anesthesia was performed to assess residual tumor, and documented in the form of clinical diagram. A trans-rectal bi-planar probe (BK Medical Flex Focus 800, probe No. 8848) covered with sterile gel inside a plastic sheath was inserted into the rectum and set at a frequency of 6 Hz. During longitudinal scan, the probe was inserted about 10 cm into the rectum, and the ultrasonic depth was set at 5.6 cm. An appropriate intra-uterine tandem of desired length and angle was selected and inserted into the uterus under TRUS guidance. After insertion, the probe was withdrawn about 5 cm to perform transverse scanning of the tandem at the level of cervix (corresponding to flange). Residual disease (hypoechoic) in the cervix and parametrium was visualized, and maximum width and thickness of the target were measured in axial plane. Based on target geometry and target dimensions on TRUS, pre-planning was done to determine the number of needles required on each side and position of the needles to be inserted. Ovoids were pre-loaded with blunt tip plastic needles and inserted into the vagina (Figure 3). The bladder was emptied before needle insertion to prevent the needles from going into the bladder. After fixation of the applicator, the interstitial needles were advanced one-by-one under TRUS guidance to achieve optimal coverage of the target and prevent the bladder or bowel perforation. These needles were then secured with guide tubes to avoid movement. The vagina was tightly packed using betadine-soaked gauze, and a T-bandage was applied to secure the applicator in place (Figure 4). All implants were performed by a single radiation oncologist to minimize variability in the treatment.
Brachytherapy contouring, planning, and treatment details
After the procedure and recovery from anesthesia, the patient was shifted for the planning CT. The bladder was instilled with 30 cc of diluted contrast, and CT images of 3 mm slice thickness were acquired (Philips Big Bore CT) and transferred to treatment planning system. High-risk clinical target volume (HR-CTV) was contoured based on clinical examination and TRUS-based measurements at the time of brachytherapy. HR-CTV was contoured to encompass lower two-thirds of the uterine cavity. The lower border covered the inferior cervical margin. None of the patients had vaginal involvement at the time of brachytherapy, therefore the vagina was not included in HR-CTV. Intermediate-risk clinical target volume (IR-CTV) was defined by adding 1 cm margin to HR-CTV laterally and cranio-caudally, and 5 mm in AP direction, and edited from OARs. Organs at risk included the bladder, rectum, and sigmoid. After contouring, applicator reconstruction and needle digitization were performed. Point A was marked on both sides, and a conventional plan was created using standard loading. This plan was modified by loading the interstitial component to 10-20% of the tandem loading to achieve optimal target coverage and minimize doses to OARs.
The dose delivered with brachytherapy was 21 Gy in 3 fractions, 7 Gy per fraction, 6 hours apart, over two days. The GEC-ESTRO recommendations for image-guided brachytherapy were followed to achieve a D90 EQD2 to HR-CTV of 100% and D2cc of the bladder, rectum, and sigmoid to less than 85 Gy, 75 Gy, and 75 Gy, respectively. The implant quality parameters analyzed were the ability to insert the tandem, ratio of the needles loaded to the number of needles inserted, and the incidence of uterine or OARs perforation. The dosimetric parameters analyzed included TRAK, dose to point A* (14 × TRAK), D90 HR-CTV, D98 HR-CTV, D98 IR-CTV, and D2cc to OARs (bladder, rectum, and sigmoid).
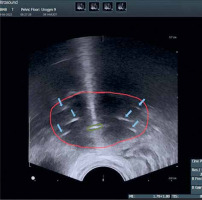
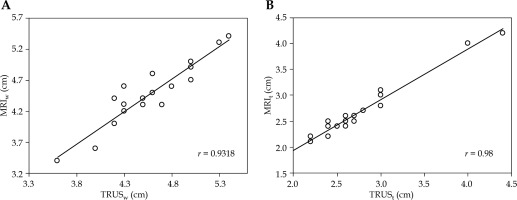
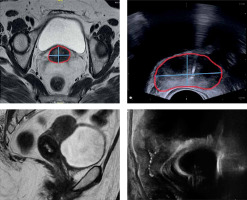
The maximum width and thickness of the target were compared between MRI before brachytherapy (MRIw and MRIt) and TRUS images at the time of applicator insertion (TRUSw and TRUSt) (Figure 5). For TRUS, after insertion of the tandem, the TRUS probe was advanced till the flange and then serially upwards to determine axial section with maximum target width. This section image was applied for TRUS-based target measurements. For pre-brachy MRI, the serial axial sections were analyzed from cervical os upwards (corresponding to flange), and the slice with maximum target width was applied for measurement. We assumed this method would provide reasonable accuracy, as the target dimensions remain the same irrespective of whether the applicator is inserted during imaging or not.
Fig. 5
Axial and sagittal image of a patient depicting width and thickness of the target measured on TRUS and MRI
All MRI and TRUS data sets were evaluated by one observer to avoid inter-observer variability. To reduce potential bias, the maximum width of the tumor was measured first on TRUS during brachytherapy procedure. This information along with clinical examination findings was used to contour HR-CTV on CT images. MRI images before brachytherapy were then analyzed to assess MRIw and MRIt, and documented.
Applicator insertion and removal-related acute side effects
Applicator insertion-related acute side effects documented were pain and vaginal bleeding. Pain was evaluated once the patient was shifted from anesthesia recovery for planning scan and at the time of implant removal. Numeric rating scale (NRS) was used to categorize the pain as painless (0 point), mild (1-3 points), moderate (4-6 points), and severe (7-10 points), based on patient description. Vaginal bleeding was recorded at the time of implant removal, and was categorized as mild (no vaginal packing required), moderate (vaginal packing required), and severe (vaginal packing and hemostatic drug required with or without blood transfusion).
Statistical analysis
A convenience sample size of 20 patients was chosen for analysis. MRIw and MRIt, and TRUSw and TRUSt were documented in centimeters and expressed as mean (± standard deviation [SD]). The comparison and correlation of different imaging dimensions and volumes of target and OARs were analyzed using Pearson’s correlation coefficient test. Pearson’s coefficient (r) values of 1 to 0.7, < 0.7 to 0.5, and < 0.5 were considered strong, moderate, and poor, respectively.
Results
The present study was conducted from October 2021 to March 2022. A total of 20 patients diagnosed with cervix carcinoma and treated with radical EBRT along with weekly chemotherapy (inj. cisplatin 40 mg/m2), followed by brachytherapy boost using hybrid IC/IS technique, were prospectively included for analysis. Clinico-pathological characteristics of the patients are presented in Table 1.
Table 1
Clinico-pathological characteristics of patients treated with TRUS-guided IC/IS brachytherapy (n = 20)
Implantation was performed under TRUS guidance using Fletcher style tandem and ovoid applicator with interstitial component. The procedure duration was 30-45 minutes. The median number of needles inserted were 6 (range, 2-10 needles). A total of 136 needles were inserted in 20 implants, of which 128 needles were loaded (94%). Details of the implant procedure quality parameters are described in Table 2.
Table 2
Details of TRUS-guided IC/IS brachytherapy implant procedure (n = 20)
The HR-CTV was contoured on the planning CT images based on clinical examination at the time of brachytherapy and TRUS-based target dimensions. We included the lower two-thirds of the uterus in the HR-CTV, but we did not analyze the height on TRUS imaging. The mean HR-CTV width (±SD) at brachytherapy was 4.58 cm (±0.44) on TRUS and 4.49 cm (±0.50) on MRI. The mean HR-CTV thickness (±SD) at brachytherapy was 2.7 cm (±0.59) on TRUS and 2.62 cm (±0.59) on MRI. On statistical analysis, there was a significant correlation between TRUSw and MRIw (r = 0.93), and TRUSt and MRIt (r = 0.98) (Figure 6A, B).
The mean HR-CTV volume was 36 cc (range, 3.2-56 cc). All patients received a brachytherapy dose of 21 Gy in 3 fractions, 7 Gy per fraction, 6 hours apart in a single session. The planning aims were to achieve a D90 to the HR-CTV of 100%. The dosimetric parameters of IC/IS plans are described in Table 3. The mean D90 HR-CTV and D98 HR-CTV were 87.3 Gy and 82 Gy EQD2, respectively. The mean D2cc to the bladder, rectum, and sigmoid were 80 Gy, 70 Gy, and 64 Gy EQD2, respectively. The bladder D2cc was less than 85 Gy in 90%, and the rectum D2cc was less than 75 Gy in 96% of hybrid IC/IS plans. None of the patients received D2cc more than 75 Gy to the sigmoid in hybrid IC/IS plans.
Table 3
Dosimetric parameters of TRUS-guided IC/IS plans
Post-procedure, 14 patients (70%) had mild pain, and 6 (30%) patients had moderate pain after recovery from anesthesia. All patients were administered analgesics till the implant was removed. Nine patients (45%) had mild pain, and 11 (55%) patients had moderate pain during implant removal. Vaginal bleeding was mild in 16 (80%) patients, and moderate in 4 (20%) patients after implant removal. The bleeding was controlled in all patients with vaginal compression or vaginal packing. None of the patients had severe bleeding post-implant removal warranting blood transfusion.
Discussion
Our study demonstrated excellent coverage of the target with TRUS-guided hybrid IC/IS brachytherapy. The number of needles to be inserted and the orientation of the needles was based on the target dimensions on TRUS at the time of applicator insertion. The length of the needles to be inserted inside the target was also based on TRUS sagittal views to minimize organ perforation. Using TRUS for applicator geometry improves the target coverage significantly and reduces the risk of organ perforation. Lin et al. have analyzed the application of TRUS in guiding interstitial brachytherapy for cervical cancer [9]. They compared the accuracy of TRUS-guided needle insertion to MRI-guided insertion in interstitial brachytherapy using Utrecht applicator, and concluded that TRUS showed strong agreement with MRI in determining the position of the inserted needles.
The dose to HR-CTV is the single most important prognostic factor for local control in carcinoma cervix [11]. The mean D90 to the HR-CTV in our study was 87.3 Gy, and the mean doses to OARs were within the GEC-ESTRO image-guided brachytherapy recommendations [2]. The tandem insertion rate was 100%, and none of the patients had uterine perforation. Two patients had bladder and bowel perforations, which were visible on CT imaging at the time of contouring. The needle tips went inside the OARs after passing through the target. Care was taken not to load the length of the needle inside the OARs at the time of planning.
The two major factors that determine dose to HR-CTV are the implant geometry and accuracy of target delineation. Hybrid brachytherapy applicators have a relatively predictable implant geometry compared with perineal interstitial templates. Based on pre-brachytherapy MRI and clinical examination, pre-planning is performed to determine the number of needles and the position of needles to be inserted at the time of the procedure, in order to achieve optimal target coverage. In complex scenarios, an MRI is performed with an applicator in situ to determine the position and length of needles to be inserted [12]. In high volume centers with resource limitations, it is not possible to perform MRI imaging with applicator in situ at the time of procedure. The ability to perform an MRI before brachytherapy is also limited in developing countries due to financial constraints. Implant geometry may not be similar to that expected on pre-planning due to incorrect tandem insertion or anatomical variations, such as deviated os and narrow vagina. TRUS imaging helps in real-time visualization of the target, and guides tandem and needle insertion, which eliminates the need for pre-planning and improves implant geometry significantly.
The IBS-GEC ESTRO-ABS recommendations for CT-based contouring in image-guided adaptive brachytherapy for cervical cancer were published recently to improve the quality of brachytherapy contouring using alternate imaging modalities [13]. Trans-abdominal ultrasound can be utilized to delineate the outer and inner dimensions of the uterine cervix and corpus, with accuracy comparable to MRI [14, 15]. However, the accuracy for parametrium evaluation is sub-optimal. Additionally, abdominal ultrasound-guided needle insertion is susceptible to hindrance caused by factors, such as intestinal gas and obesity. TRUS-guided implant insertion and incorporating TRUS-based measurements for CT-based target volume contouring can significantly improve outcomes in centers with limited access to MRI [3, 16, 17].
Mahantshetty et al. have described the concept of “near maximum distance” (NMD), which is a measurement of the lateral extension from the cervical canal (os), and needs to be specified for each parametrium (left and right) [13]. TRUS-based NMD’s at brachytherapy can aid in defining and contouring HR-CTV (in asymmetrical targets), and in determining the type of BT application (IC alone/IC + IS) and needle placement to be performed.
Our study showed that there was no significant differences between TRUS and MRI for the assessment of HR-CTV width and thickness. The mean differences and standard deviations were in a comparable range to studies, which have compared TRUS and MRI for target delineation in carcinoma cervix brachytherapy (Table 4) [3, 22]. CT, in contrast, yields significantly higher deviations, systematically overestimating the HR-CTV width in all patients, as previously published in literature [18, 19].
Table 4
Comparison of studies documenting correlation between TRUSw and MRIw, and TRUSt and MRIt for defining target during brachytherapy in carcinoma cervix patients
| Study | Number of patients | Mean difference of TRUSw and MRIw (cm) | Mean difference of TRUSt and MRIt (cm) |
|---|---|---|---|
| Schmid et al. | 17 | 0.0 ±0.3 | –0.2 ±0.3 |
| Mahantshetty et al. | 25 | 0.5 ±0.6 | 0.2 ±0.6 |
| Present study | 20 | 0.2 ±0.4 | 0.1 ±0.1 |
IC/IS applicator insertion for brachytherapy is an invasive procedure, and can cause patient discomfort and pain during the entire course of treatment. The factors that affect the severity of pain and bleeding post-applicator insertion are the number of needles inserted, the depth, to which the needles were inserted, and perforation of organs at risk [20]. The median number of needles used in each insertion in our patients was 6. The median depth, to which the needles were inserted beyond the vaginal ovoids was 4 cm. These factors combined with low incidence of OARs perforation reduced patient discomfort. None of the patients had severe pain, and majority of the patients experienced mild pain post-implant insertion. Vaginal bleeding after applicator removal was mild in most of the patients. Patients having moderate vaginal bleeding were managed with vaginal packing, and none of the patients required hemostatic drugs or blood transfusion post-implant removal. Sharma et al. reported outcomes of TRUS-guided interstitial brachytherapy in cervical cancer using MUPIT template [10]. They concluded that TRUS assists in avoiding the needle injury/perforation of pelvic structures very accurately and precisely, thereby reducing the risk of peri-operative complications.
The EMBRACE investigators compared intra-cavitary vs. hybrid IC/IS applicators (both Vienna and Utrecht style), and found that hybrid ring IC/IS applicators provided the best therapeutic ratio [21]. However, there is a potential learning curve utilizing a new applicator, and optimal applicator insertion requires expertise in terms of the number of needles to be inserted and how far to insert them. There is limited data regarding TRUS-guided imaging for hybrid applicator insertion and TRUS-based target volume contouring. The ability to achieve the optimal target coverage and reduced doses to OARs is an indicator of ideal implant geometry accomplished with TRUS guidance in our study. These findings, along with a good correlation between TRUS and MRI-based target dimensions, highlight the importance of TRUS in cervix brachytherapy. One limitation of our study is that it is a dosimetric study with a small sample size. These patients require follow-up to analyze the outcomes of TRUS-guided IC/IS hybrid brachytherapy. As we do not perform MRI at brachytherapy, we could not compare the two modalities, i.e., MRI and TRUS, with applicator in situ. This would have made the comparison more accurate. Also, image acquisition with TRUS is not as precise as MRI in case of significant parametrial disease or extensive vaginal involvement at brachytherapy. Another limitation of our study is that we included the lower two-thirds of the uterus in HR-CTV, as TRUS is not an accurate tool for measuring uterine involvement. If there is no uterine involvement on diagnostic MRI, the upper limit of HR-CTV can be reduced. But for centers, in which it is not possible to perform an MRI at diagnosis and before/during brachytherapy, this practice can be adapted. We believe the present study can serve as a useful guide for centers willing to incorporate TRUS imaging to guide applicator insertion and measure target dimensions. This would improve treatment outcomes and reduce toxicity in high volume centers with resource limitations.