Purpose
Skin cancer is the most common malignancy especially in elderly patients [1]. Several researches estimate that non-melanoma skin cancer (NMSC) affects more than 3 million Americans a year [2, 3]. The two most common types of NMSC are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), with BCC (75%) representing majority of NMSC [4-6]. For BCC, the main carcinogenic factor is ultraviolet light (UV) exposure, which explains why most tumors are located on sun-exposed sites, and the risk of developing BCC for white-skinned people is about 30% [7]. For SCC, other risk factors are precursor lesions, including actinic keratosis and SCC in situ [6]. Surgical excision is often the primary treatment for non-melanoma skin cancer, with reported < 5% local recurrence rates. Moreover, other loco-regional approaches, such as radiotherapy, cryotherapy, and photodynamic therapy, are also used. Radiotherapy (RT), both external beam and brachytherapy, is considered as definitive treatment for patients who are unfit for surgery (locally advanced disease, comorbidities, or refused surgery), or for patients > 60 years with non-sclerodermiform histology and lesions in sites where curative surgery pose a significant risk of poor aesthetic outcome. A tailored dose of 60 to 70 Gy is usually prescribed [8, 9]. For SCC with poor prognostic factors or when excision is incomplete and re-excision is not feasible, adjuvant RT is considered an option [10].
High-dose-rate (HDR) brachytherapy (BT) plays an important role in the treatment of NMSC regarding adaptability, patient’s safety, and great flexibility of dose fractionation, which allows to obtain excellent rates of care and cosmetic results [11]. Since there are no radiosensitive areas in a superficial skin field, and due to regenerative capacity of the skin, hypofractionated radiation therapy is often applied for superficial skin tumors [12]. Moreover, no significant data on hypofractionation related to worse cosmesis exist. Also, longer treatment times are unfit for elderly patients and are not cost-effective.
Valencia applicators are accessories of a remote afterloader MicroSelectron HDR (Nucletron B.V., Veenendaal, The Netherlands) [11, 13-15], provided with a flattening filter. This filter produces flat dose-rate distributions with a small dim light to deliver radiation to target volume and saving surrounding normal tissues [16]. The applicators are produced of tungsten, with two sizes of 20 mm and 30 mm in diameter, and are used for treating skin lesions up to 30 mm in diameter and 4 mm deep [16, 17].
The aim of this study was to retrospectively analyze tumor control, toxicity, and aesthetic events in NMSC patients treated with iridium-192 (192Ir)-based HDR-BT using Valencia applicators.
Material and methods
In this retrospective study, data of 95 patients affected by NMSC and treated with Valencia applicators 192Ir-based HDR-BT at the Division of Radiotherapy, University of Pisa, from June 2015 to December 2020 were examined. Data, including hospital records, pathological reports, radiotherapy modalities, and follow-up information were recorded.
Patients over 18 years old of age, with newly diagnosed, histopathologically-proven BCC or SCC of the skin were included in this study. First, skin biopsy was done to confirm the diagnosis, and to measure tumor depth with Breslow rate. In some selected cases, high frequency ultrasonography (HFUS) was performed to measure tumor depth.
In total, 182 lesions with a depth ≤ 4 mm, measuring ≤ 25 mm in diameter (median, 12 mm; range, 3-25 mm) were analyzed. Valencia applicators are accessories of remote afterloader MicroSelectron HDR (Nucletron B.V., Veenendaal, The Netherlands) that are made of tungsten and equipped with flattening filter [11, 16]. Usually, gross tumor volume (GTV) was visually assessed. Lesions with sizes of 20 mm or 30 mm in diameter were selected to allow a margin of at least 5 mm from the edge of the field.
Methods of immobilization, such as customized headrests or tapes were usually employed. High accuracy of tumor depth measurement is required for high-dose gradient; therefore, RT dose was prescribed at 3 mm for lesions with a depth ≤ 3 mm and at 4 mm for those with a depth ≤ 4 mm.
Prescription dose of 40 Gy was delivered in 8 fractions/5 Gy [biological effective dose (BED) ≈ 60 Gy], 2-3 fractions a week, with a minimum interval of 24 hours between fractions.
The equivalent dose in 2 Gy fractions (EQD2) was calculated by using the following formula:
where D is the total dose in Gy, d is the dose per fraction in Gy, and the α/β ratio is considered 10 Gy for the tumor [17].
High-dose-rate BT was delivered with a 192Ir source using MicroSelectron Elekta HDR afterloader. Generally, each radiotherapy daily fraction must be administered in the presence of BT-experienced radiotherapist, who supervise the accuracy of procedures and dose delivery by constant monitoring of the treatment using video camera and audio connection with the treatment room.
Acute and late toxicities were evaluated according to common terminology criteria for adverse events (CTCAE) v. 5.0 [18]. Cosmetic results were assessed at each follow-up visit according to the Radiation Therapy Oncology Group-European Organization for Research and Treatment of Cancer (RTOG-EORTC) scale [19]. Follow-up visits were scheduled every 3 to 4 months for the first 2 years after BT completion, then every 6 months for the next 3 years, and once a year after 5 years. The results are presented as median or mean values for quantitative parameters. Frequencies and percentages were calculated for qualitative parameters. Local control (LC) was evaluated with Kaplan-Meier method. All patients were periodically followed-up, until December 2020 or their death.
Results
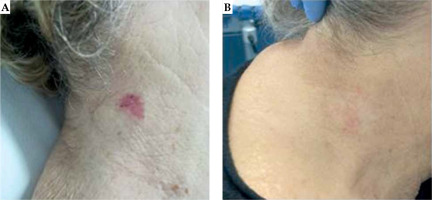
Patients and lesions characteristics are shown in Table 1. For surface treatment, 105 (57.7%) applicators with a diameter of 20 mm, and 77 (42.3%) applicators with a diameter of 30 mm were used. One hundred and sixty-nine lesions (92.9%) received a radical treatment, and thirteen lesions (7.1%) underwent adjuvant BT after surgery. One hundred and seventy-seven lesions (97.3%) achieved a complete response (CR) (Figure 1), and 5 lesions (2.7%) had a partial response (PR) at clinical evaluation performed three months after completion of treatment.
Table 1
Patients and lesions characteristics
Fig. 1
A) Example of complete treatment response of basal cell carcinoma of the right neck using high-dose-rate brachytherapy and Valencia applicator of 30 mm in diameter. B) At three months after treatment
All five PR lesions and two CR (1.1%) lesions presented local progression after a median time of 10 months (range, 6-23 months). Regarding disease progressions, three PR (1.6%) lesions that were histologically-proven SCCs, had local and nodal progression, and were treated with palliative external beam radiotherapy (EBRT) and chemotherapy. Two other PR (1.1%) lesions were histologically-proven BCCs of the face, and underwent surgery. Two CR (1.1%) BCC lesions of the face and the nose were treated with surgery and chemotherapy. No patient developed distant disease. Only three PR patients (3.1%) died of the disease, other deaths were due to age and/ or comorbidities. The median follow-up of survivors was 14 months (range, 3-59 months). The 2-year local control rate was 96%. All the patients completed radiation treatment, and BT was well tolerated. No treatment was stopped due to toxicity.
As shown in Table 2, the most common G1 toxicities were dermatitis (16.0%, n = 29) and pain (8.2%, n = 15), and G2 acute toxicity was dermatitis only (6.0%, n = 11). The most common G1 late toxicities were hypo-pigmentation (27.5%, n = 50) and fibrosis (8.2%, n = 15), and the only G2 late toxicity was ulceration (0.5%). No G3 or higher acute and late toxicity were observed. Excellent cosmetic results were noted in 77.5% of lesions (n = 141), and only one treated lesion (0.5%) presented a poor cosmetic result (ulceration refractory to therapy) (Table 3).
Table 2
Acute and late toxicities (CTCAE v. 5.0) of non-melanoma skin cancer (NMSC) patients who underwent 192Ir-based HDR-BT using Valencia applicators
Discussion
All treatment options in NMSC patients have as ‘target’ cancer treatment, with preservation of function and optimization of cosmesis. The primary modality for NMSC is surgical excision, with histological control of excision margins and recurrence rates for BCC from 2% to 8% at 5 years, as reported by Trakatelli et al. [20]. Radiotherapy may be considered the primary treatment in patients unfit for surgery, including those with tumor site or locally advanced disease. Patient’s age, poor performance status, comorbidities, or refused surgery are additional factors. Moreover, RT could damage surrounding normal tissues, resulting in toxicity or aesthetic changes, which are most sited within radiation field [9].
Several radiotherapy techniques, including superficial X-rays, orthovoltage X-rays, megavoltage X-rays, electron beam irradiation, low-dose-rate (LDR) BT, HDR-BT, external beam radiotherapy (EBRT), and recently, electronic brachytherapy, are used to treat skin cancer [21, 22]. Surface BT is a non-invasive therapeutic approach that uses applicators (Leipzig and Valencia applicators). These applicators, which are placed directly on the body surface and precisely on tumor site, are used to treat superficial and well-defined lesions. The advantages of HDR-BT with Valencia applicators are swift and safe dosimetric calculations, and easy reproducibility of patients positioning in order to avoid procedural mistakes [11].
According to literature [23-25], in our study, HDR-BT seemed to be a non-invasive and effective therapeutic approach for NMSC, with poor high-grade toxicities. Also, cosmetic results of the patients included are very promising, with more than 96% of the cases showing good/excellent results detected at follow-up. Taylor et al. [26] observed grade 2 toxicity at completion of treatment in 85% of patients and no grade 3 or higher skin toxicity. Moreover, Gauden et al. in a cohort of 200 patients reported G1 acute skin toxicity in 168 treated lesions (71%), G2 in 81 (34%), and good or excellent cosmesis in 208 cases (88%). Late skin hypopigmentation was observed in 13 (5.5%) patients [27].
Several studies, in which HDR-BT was compared with surgery showed that RT provided lower local control [26, 28, 29]. Indeed, some surgery studies showed recurrence rates at 5 years < 5%, but also certain research on RT demonstrated LC rates between 95% to 100%. Taylor et al. [26] reported a LC rate of 95% with a median follow-up of 7.2 months (range, 1.3-54.3 months), and in a review, Delishaj et al. [30] described a median LC of 97% in HDR-BT study. Moreover, Gauden et al. [27] compared LC of HDR-BT-treated patients (98%) with LC of EBRT-treated patients, and reported both LC rates from 87% to 100%, respectively, with a follow-up from 2 to 5 years. The current research reported 2-year local control rate of 96%, according to other BT studies. Lower LC rates may be caused by tumor sizes (small vs. large), sites (plain vs. curved surfaces), margins status, and histology (BCC vs. SCC) [24, 27, 30-32]. Here, we found no statistically significant difference in LC rates between the two histological groups of patients (data not shown).
In our study, no statistically significant difference between definitive and adjuvant treatment groups (data not shown) was observed. In some cohorts of elderly patients, surgical resection was associated with higher complications and worse results [26, 30]. According to the literature, surface HDR-BT is considered particularly tailored for elderly population, often with poor performance status and/or concomitant comorbidities; therefore, often unfit for surgery or longer RT.
In conclusion, our results suggest that HDR-BT using Valencia applicators is a safe and effective therapeutic approach in NMSC patients. BT is a well-tolerated treatment with insignificant high acute and late toxicities and good cosmesis. Moreover, brachytherapy is the recommended therapeutic modality for elderly patients in particular, because it is associated with better outcomes and compliance.


