In general, critically ill patients are susceptible to vascular thrombosis and have a higher risk of bleeding compared to non-critically ill medical or surgical patients. The advent of direct oral anticoagulants (DOACs) is a revolution in medicine. Unfortunately, clinical trials of the use of DOACs in the intensive care unit (ICU) are lacking. Over the last decade, the utilization of DOACs has increased, and critical care clinicians can come across patients with known and unknown use of DOACs [1]. Pivotal phase III DOAC trials did not include patients requiring ICU management, patients with acute kidney injury, patients with major bleeding, or patients in need of urgent surgical interventions [2–6].
This narrative review covers current evidence on the use of DOAC in indicated conditions and anticoagulant management of medical or surgical patients receiving DOAC before ICU admission. This article is the second part of the narrative review. Pharmacology, including indications and dosing, has been covered in the first part: “Use of direct oral anticoagulants in ICU patients. Part I – Applied pharmacology”.
SEARCH STRATEGY AND SELECTION CRITERIA
This is a non-structured narrative review to provide current clinical evidence about the use of DOACs in ICU patients. The review was conducted by a search on the PubMed, Embase, Medline, Google Scholar, and Cinicaltrials.gov databases. The search included publications from 1994 to 2020, using the terms “dabigatran”, “rivaroxaban”, “apixaban”, “edoxaban”, or “betrixaban”, “oral anticoagulants use in critically ill patients”, “DOACs/NOACs use in the intensive care unit”, “oral anticoagulants in sepsis, shock, and acute kidney injury”, and “oral anticoagulant management in the perioperative period” to identify relevant papers, including randomized clinical trials, meta-analyses, observation studies, case series, and guidelines.
CURRENT EVIDENCE FOR USE OF DOAC
Atrial fibrillation
Warfarin (vitamin K antagonist) remains the anticoagulant of choice for patients with valvular atrial fibrillation (AF), secondary to mechanical valves and moderate to severe rheumatic mitral valve stenosis, due to the exclusion of such patients from phase III DOAC trials and evidence of thrombotic and bleeding risks in these patients [7–10]. Current guidelines recommend using DOACs in patients with non-valvular AF (NVAF) because DOACs are non-inferior to warfarin in stroke prevention in this population [11, 12]. Due to the lack of evidence and pragmatism, the consensus opinion is to switch a DOAC to a parenteral anticoagulant if such patients are admitted to the ICU either due to AF, AF-associated complications (e.g. stroke), the need for an invasive procedure, or sepsis/shock [13, 14]. In patients with new-onset NVAF of > 48 hours, who need urgent cardioversion, DOACs can be started 4 hours before transoesophageal echocardiogram and cardioversion, if there are no other concerns related to the use of DOACs (e.g. sepsis, stroke, acute kidney injury) [11, 15]. Patients with NVAF of < 48-hour duration who need urgent cardioversion can receive DOACs as soon as possible before or immediately after cardioversion [11, 12, 15].
Patients with bio-prosthetic valves were also excluded or minimally included in DOAC trials [3–6]. Current guidelines are not clear about using DOACs for stroke prevention in patients with AF and bioprosthetic valves; such patients could be eligible for DOACs after 3 months of surgery [11]. But, as per the manufacturers of DOACs, such use is considered off-label and is not recommended [16–18].
Acute coronary syndrome
Patients already on a DOAC/Patients with a new indication for anticoagulation
Parenteral anticoagulants (unfractionated or low-molecular-weight heparin, bivalirudin, fondaparinux) are the recommended agents during the management of acute coronary syndrome (ACS) [19, 20]. For patients already taking DOACs, who present with ACS, DOACs should be stopped on admission [13]. For ST-segment elevation myocardial infarction (STEMI) patients undergoing percutaneous coronary intervention (PCI), parenteral anticoagulation is initiated as soon as possible, regardless of the timing of the last dose of DOAC [14, 20]. Fibrinolysis can be considered if PCI is not available and fibrinolysis is the only option, and if the plasma level of a specific DOAC is below the reference range and DOAC-related coagulation parameters are normal (as discussed in part 1 of this review) [14].
For non-STEMI patients who are not in urgent need of reperfusion, parenteral anticoagulation can be delayed to dissipate the anticoagulant effect of the DOAC [11].
After management of ACS, DOAC should be resumed combined with dual antiplatelet therapy (DAPT) at least in the immediate period [11]. For patients who have a new indication for anticoagulation (atrial fibrillation, venous thromboembolism), DOAC can be started in combination with DAPT as soon as there is no further indication of parenteral anticoagulants. The total duration of triple therapy should be individualized based on the specific indication, risk of a recurrent ischaemic event, stent thrombosis, and bleeding risk [14, 21]. See Figure 1 for the practical approach.
DOAC use in ACS patients not undergoing PCI and without other indications for anticoagulation
ACS patients who do not undergo PCI are at high risk of recurrence of symptoms due to thrombus persistence, increased platelet reactivity, and elevated thrombin generation, which provides the rationale for chronic anticoagulation in such patients [22]. Rivaroxaban is the only DOAC that has been shown to reduce the risk of recurrent ischaemic events and mortality when added to DAPT in ACS patients who have not received PCI and do not have other indications for anticoagulation (e.g., AF or VTE) [23, 24]. Rivaroxaban was used at a much lower dose (2.5 mg or 5 mg twice daily) compared to its dose for AF patients (20 mg once daily), and it was noted that a 5 mg dose did not show a survival benefit [24]. Based on the phase III ATLAS ACS TIMI 51 trial (Anti-Xa Therapy to Lower Cardiovascular Events in Addition to Aspirin With or Without Thienopyridine Therapy in Subjects with Acute Coronary Syndrome–Thrombolysis in Myocardial Infarction 51), rivaroxaban was recommended in combination with aspirin or aspirin plus clopidogrel (or ticlopidine) in certain ACS patients with a low bleeding risk [21, 24].
Dabigatran or apixaban combined with DAPT led to increased bleeding risk without reducing ischaemic events [23, 25]. Importantly, randomized trials are ongoing to assess the safety and efficacy of DOACs when used in combination with antiplatelets [26, 27].
Post cardiac valve replacement
Warfarin remains the anticoagulant of choice in patients with mechanical valves [11, 12]. In the recent past, there has been a shift from mechanical to bioprosthetic valves [28]. Even though bioprosthetic valves are less thrombogenic than mechanical valves, the true incidence of bio-prosthetic valve thrombosis is more than previously thought [28]. Recommendations regarding antithrombotic prophylaxis following bioprosthetic valve implantation remain controversial regarding the adequacy of antiplatelet therapy alone versus the addition of either warfarin or a DOAC, especially in the early post-procedure period. Warfarin is also the agent of choice for bio-prosthetic mitral valve and mitral valvuloplasty [15]. Patients who undergo transcatheter aortic valve replacement and have AF could use DOACs after 3 months of the procedure as per the European Society of Cardiology [11]. However, the American Heart Association (AHA) and DOAC manufacturers do not recommend such use [15–18].
Venous thromboembolism treatment (deep venous thrombosis and pulmonary embolism)
DOACs have been approved for the treatment of venous thromboembolism (VTE) [29]. DOACs are non-inferior to low-molecular-weight heparin (LMWH) and warfarin to prevent recurrent VTE [30–32]. Patients with haemodynamic compromise or those with the anticipated need for invasive intervention should be treated with unfractionated heparin during the critical phase. DOACs can be started as soon as there is no need for invasive intervention and patients are haemodynamically stable [29].
Patients on DOACs can have a recurrent VTE [33, 34]. In such cases, an incorrect dose of DOAC, non-compliance, and any drug interactions should be excluded. Such cases can be managed by switching to full-dose LMWH for at least 1 month [34, 35].
There is a lack of data to guide clinicians whether to use thrombolysis in patients who develop pulmonary embolism (PE) while taking a DOAC and require thrombolysis due to haemodynamic compromise. It may be reasonable to consider thrombolysis in cases of arrest or peri-arrest due to PE in the setting of concomitant DOAC use [1]. Endovascular intervention could be an option in patients with intermediate to high risk [1].
Venous thromboembolism prevention
VTE incidence has been reported to be as high as 40% in critically ill patients who do not receive thromboprophylaxis [36]. Trials have evaluated the use of DOACs for VTE prevention in hospitalized patients with acute medical illness, but not in ICU patients [13]. Betrixaban is the only DOAC approved with an extended duration of prophylaxis against VTE in medically ill hospitalized patients, but current guidelines do not recommend extended duration thromboprophylaxis [37, 38].
Acute ischaemic stroke
Data on the safety and efficacy of intravenous (iv) thrombolysis in ischaemic stroke patients taking DOACs is minimal [39]. The American Heart Association (AHA), American Stroke Association (ASA), and European Heart Rhythm Association (EHRA) discourage the use of IV thrombolytics in patients presenting with acute ischaemic stroke who are on DOACs [14, 40]. As per AHA/ASA/EHRA, IV thrombolytics may be considered if the DOAC plasma level is below the detection limit and DOAC-related coagulation parameters are normal, or if the last dose of DOAC was > 48 hours before presentation in a patient with normal renal parameters [14, 40]. Endovascular therapy may be considered for patients who are not candidates for IV thrombolysis due to the use of DOACs [11].
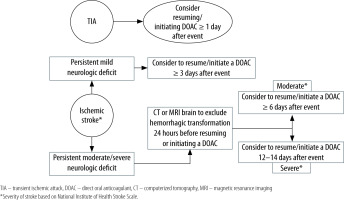
Current evidence is not sufficient to guide with certainty when to resume or initiate DOACs (in the setting of AF) after ischaemic stroke [3–6]. So far, only prospective observational studies and 2 small, randomized trials have assessed the timing, efficacy, and safety of DOACs following a cardioembolic stroke [41–44]. These studies have reported that early DOAC administration (with a median of 3–5 days) in mild to moderate AF-associated ischaemic stroke did not increase symptomatic or radiographic haemorrhage, but delayed administration was associated with increased frequency of recurrent ischaemic stroke [41–44]. AHA and ASA recommend starting anticoagulation after 4–14 days of ischaemic stroke in the setting of AF [45]. EHRA suggests a timeline to resume or initiate anticoagulants based on the severity of stroke/neurologic deficit. DOAC can be resumed or started 1 day after a transient ischaemic attack, ≥ 3 days after mild ischaemic stroke, ≥ 6–8 days after moderate stroke, and ≥ 12–14 days after severe stroke [14]. In patients with moderate and severe stroke, haemorrhagic transformation should be excluded before starting an anticoagulant [14]. See Figure 2 for a practical approach.
Heparin-induced thrombocytopaenia
In vitro analyses have demonstrated that DOACs do not activate platelet aggregation in the presence of antiplatelet factor 4/heparin antibodies [46]. Current evidence evaluating the use of DOACs to manage heparin-induced thrombocytopaenia (HIT) is limited to small retrospective studies. The acuity of the studied population, if reported, was low, which prohibits the application of results to critically ill patients [47, 48]. Based on current evidence, the American Society of Hematology 2018 guidelines included conditional recommendations to use DOACs in the acute phase of HIT, but only in clinically stable patients with an average risk of bleeding [49].
Cerebral venous thrombosis
Due to the significantly reduced risk of intracranial haemorrhage compared to warfarin, DOACs are an attractive option for the long-term treatment of cerebral venous thrombosis (CVT) [50, 51]. A multicentre prospective observational study by Wasay et al. [52] found that using a DOAC (rivaroxaban or dabigatran) for the long-term treatment of CVT is safe and as effective as warfarin. In this study, patients were treated with UFH or LMWH during the acute phase, and an oral anticoagulant (either DOAC or warfarin) for the long-term treatment was started within a median of 7 days of diagnosis of CVT. Recently published results of the RE-SPECT CVT trial (Rationale, design, and protocol of a randomized controlled trial of the safety and efficacy of dabigatran etexilate versus dose-adjusted warfarin in patients with cerebral venous thrombosis) showed that dabigatran is non-inferior to warfarin in treating CVT, and with fewer bleeding events [53]. Currently, warfarin is being used for the long-term treatment of CVT [54].
DOAC USE DURING CONCURRENT ACUTE MEDICAL CONDITIONS
Sepsis
Sepsis is commonly encountered among hospitalized patients and is associated with coagulation abnormalities [55]. Patients with sepsis are at high risk of multiorgan dysfunction and poor outcomes as sepsis is associated with microvascular thrombosis or bleeding due to depletion of coagulation factors and platelets [56]. Sepsis increases the risk of AF and VTE [57]. Observational data have shown that the use of warfarin in patients with AF during critical illness is associated with an increased risk of bleeding [57, 58]. Unfortunately, there are no safety and efficacy data for the use of DOACs in patients with sepsis if they need therapeutic anticoagulation [3–6]. For patients who are already on DOACs and need ICU admission due to sepsis, it is suggested to switch DOAC to UFH or LMWH [13].
Acute kidney injury
As discussed in Part I of this review series (“Use of direct oral anticoagulant in ICU patients. Part I – Applied pharmacology”), all DOACs are dependent on renal excretions to some extent. Renal impairment, either acute or chronic, affects the clearance of the DOACs [59]. In the case series by Singh et al. [60], all 5 cases of dabigatran-associated acute bleeding had an acute decline in the renal functions, and all of them were on a stable dose of DOAC before the critical events. Unfortunately, so far, there are no safety and efficacy data on DOACs in acute kidney injury patients. Switching DOACs to alternative anticoagulants is suggested for patients with acute kidney injury [14].
PERIOPERATIVE MANAGEMENT OF PATIENTS ON DOAC
Emergency surgery
Idarucizumab and andexanet alfa are the 2 agents approved by the Food and Drug Administration (FDA) to reverse the anticoagulant effect of dabigatran and factor-Xa-inhibitors, respectively. The RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) trial evaluated outcomes in patients who received idarucizumab in the setting of urgent surgical interventions or bleeding [61]. Unfortunately, the ANNEXA-4 (Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors) trial evaluated patients who had factor-Xa-inhibitor-associated bleeding and did not include patients in need of urgent surgical intervention [62].
Based on the urgency and bleeding risk of the procedure, idarucizumab and andexanet alfa can be considered to prevent bleeding complications [11, 14, 61]. If specific reversal agents are not available and surgical intervention is associated with increased risk of bleeding, prothrombin complex concentrates (unactivated or activated) and antifibrinolytics (e.g., tranexamic acid) can be considered to reduce bleeding [63–65].
Elective surgery
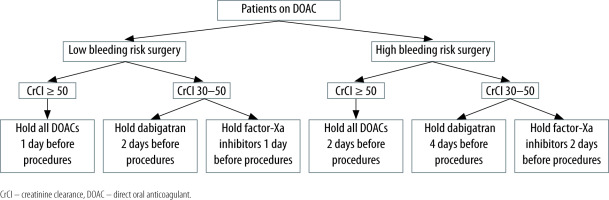
Outcomes in patients on DOACs and the need for elective procedures have been examined according to the bleeding risk of the surgery [66, 67]. Most dermatologic, dental, and ophthalmologic procedures can be performed without interruption of systemic anticoagulation, usually 24 hours after the last dose [11, 68, 69]. For other procedures, pre-procedure cessation of anticoagulants is generally recommended, which depends on the risk of bleeding, clinical impact of holding the anticoagulant, and renal clearance of the DOAC [11, 68, 69]. Commonly, DOACs are held 1–4 days before the procedure. In patients with creatinine clearance (CrCl) < 50 mL min−1, dabigatran should be discontinued at least 4 days and factor-Xa-inhibitors at least 2 days before the procedure [14, 68–70] (see Figure 3).
Due to the quick onset of action and short half-life of DOACs, peri-procedural bridging is not recommended [11, 71].
Post-procedure resumption of DOACs is based on post-procedure bleeding risks and consultation with the clinician who did the procedure. The expert opinion is to hold the DOAC for the same number of days after the procedure as before the procedure [13, 70].
Patients undergoing cardiac device implantation
Uninterrupted use of warfarin has shown better outcomes than bridging with parenteral anticoagulation in patients who need cardiac device implantation [72, 73]. Interrupted or continuous DOAC therapy during device implantation remains a matter of debate [74]. Most clinicians discontinue DOACs before device implantation based on the half-life of the DOAC and CrCl. Usually, dabigatran is stopped 24 hours before device implantation in those with CrCl > 80 mL min−1, 36 hours before in those with CrCl 50–79 mL min−1, and 48 hours earlier in those with CrCl < 50 mL min−1. Patients on apixaban, edoxaban, or rivaroxaban are advised to hold the DOAC 24 hours before the procedure [72, 74]. After device implantation, DOACs can be resumed 24 hours after the procedure in patients with high thromboembolic risk and after 3–5 days in those with increased bleeding risks [72, 74].
Managing bleeding in patients taking DOAC
Better outcomes have been reported in patients on DOACs and suffering from intracranial haemorrhage or major traumatic bleeding compared to patients who take warfarin [51, 75]. For the management of confirmed or suspected DOAC-induced bleeding, the basic principles remain the same as for any other bleeding case [76]. If possible, specific DOAC, the timing of the last dose, and the indication for DOAC use should be identified. Laboratory tests, including renal function, tests of anticoagulation (aPTT, PT), anti-factor-Xa activity for factor Xa-inhibitors, and diluted thrombin time (dTT) for dabigatran should be performed [77]. Idarucizumab can be used to reverse the anticoagulant effect of dabigatran, and andexanet alfa or 4-factor prothrombin complex concentrates are the available options for factor Xa-inhibitors associated bleeding [11, 32, 61–64]. If the last dose of DOAC was recent enough, oral activated charcoal could decrease the absorption of the unabsorbed drug [78]. Haemodialysis can be considered to remove dabigatran from circulation [60, 79] (see Table 1).
TABLE 1
Measures to manage major bleeding in patients on direct oral anticoagulants
| Measure | Direct oral anticoagulant | |
|---|---|---|
| Dabigatran | Factor-Xa-Inhibitors | |
| Laboratory tests* | CBC, CMP, PT, aPTT, dTT | CBC, CMP, PT, aPTT, anti-factor-Xa activity |
| Specific reversal agent | Idarucizumab | Andexanet alfa |
| Prothrombic complex (PCC) concentrates¶ | If idarucizumab not available, activated PCC (FEIBA) can be used 4-factor or 3-factor PCC if activated PCC are unavailable | 4-factor PCC@ 3-factor PCC if no other agent is available |
| Antifibrinolytics (e.g., tranexamic acid) | In cases of severe bleeding or if no other agents available | In cases of severe bleeding or if no other agents available |
| DOAC removal | Activated charcoal: if last dose within past 2 hours Haemodialysis: as an option of last resort | Activated charcoal: if last dose within edoxaban: 2 hours, apixaban: 6 hours and rivaroxaban: 8 hours Haemodialysis: Factor-Xa-inhibitors cannot be removed by dialysis |
| Emergent surgery | Use specific or nonspecific agents if severe bleeding anticipated | |
CBC – complete blood count. CMP – comprehensive metabolic panel, PT – prothrombin time, aPTT – activated partial thromboplastin time, dTT – dilute thrombin time, FEIBA – factor VIII activity bypassing agent.
Scoring systems such as CHA2DS2-VASc and HAS-BLED can help decide whether to resume anticoagulant or not. DOACs may be resumed 4–8 weeks after the intracranial bleed if the underlying aetiology and bleeding risks have been managed adequately [14].
ONGOING IMPORTANT TRIALS
Continued extensive research has led to the increasing use of DOACs during the last decade. Still, the safety and efficacy related to DOACs use in critically ill patients requiring ICU admission are unanswered. Several clinical trials are underway to answer some of the essential clinical queries [80] (Table 2).
TABLE 2
Some important ongoing and future clinical trials of direct acting oral anticoagulants
CORONAVIRUS DISEASE 2019 (COVID-19) AND USE OF DOAC
In the recent COVID-19 pandemic, SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2)-infected critically ill patients demonstrated dysregulated inflammation and higher thrombotic complications (up to 45% in ICU patients) [81]. Direct factor Xa inhibitors have shown beneficial effects in COVID-19 patients, with anticoagulant, anti-inflammatory, and anti-viral properties [82]. Despite multiple potential benefits of direct factor Xa inhibitors in patients with COVID-19, good clinical evidence is scarce in terms of risk benefit at present. The longer half-life of these drugs can be detrimental when urgent invasive procedures are planned in critically ill hospitalized patients or in the event of rapid worsening of renal or hepatic function.
At present (updated May 2021), no major guidelines recommend the use of DOAC, either as a prophylactic or therapeutic, in COVID-19 patients admitted to the ICU (Table 3). Only a few guidelines recommend the use of direct factor Xa inhibitors in COVID-19 patients in specific situations like non-ICU settings for extended prophylaxis, post-discharge prophylaxis in high-risk populations, and post-discharge extended therapy in confirmed VTE patients (Table 3) [83–87].
TABLE 3
Leading organizations’/societies’ recommendations for the use of direct factor Xa inhibitors in the management of COVID-19 patients (updated till May 2021)
| Organization/society [Ref] | Month/year | Consideration for the use of direct factor Xa inhibitors in COVID-19 patients | |
|---|---|---|---|
| ICU patients (either prophylactic or therapeutic) | Non-ICU setting | ||
| American Society of Chest Physician [83] | June 2020 | No recommendation | No recommendation |
| International Society on Thrombosis and Hemostasis [84] | August 2020 | No recommendation | Rivaroxaban or betrixaban in non-ICU settings for extended prophylaxis Post discharge prophylactic use in high-risk* patients (up to 30 days) and extended therapeutic use in confirmed VTE patients |
| World Health Organization [85] | January 2021 | No recommendation | No recommendation |
| American Society of Hematology [86] | February 2021 | No recommendation | No recommendation |
| National Institute of Health [87] | February 2021 | No recommendation | Post discharge prophylactic use in high-risk* patients (rivaroxaban 10 mg daily for 31–39 days) |
* High-risk patients includes advanced age, stay in ICU, cancer, previous history of venous thromboembolism, thrombophilia, severe immobility, elevated D-dimer (> 2 times upper limit of normal), Modified International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) VTE risk score ≥ 4.
CONCLUSIONS
In the near future, it will not be uncommon for critical care physicians to have more and more patients who are already taking DOACs for various clinical conditions, due to its ever-increasing popularity because of their fixed dosing with minimal monitoring and the availability of specific reversal agents. At present, there is little evidence about the safety of continuing DOACs in critically ill patients after admission in the ICU, even if anticoagulation is indicated. In these patients, the anticoagulant effect is preferably achieved by switching to heparin (LMWH or UFH). Some studies also evaluated DOAC use for VTE prevention in hospitalized patients with acute medical illness but not ICU patients. Hopefully, ongoing and future trials will provide strong evidence for the use of DOACs in ICU patients.