Introduction
The development of medicine has allowed us to treat more complicated cases of diseases. Unfortunately, new and more effective therapies also cause new difficulties, which are frequently related to the side effects of used medicaments. Drug-induced diabetes mellitus (DIDM) is one of them.
In the 2020s we still have very little knowledge about the epidemiology and dedicated treatment of DIDM, especially in the paediatric population. Furthermore, there is no unified definition or diagnostic criteria of DIDM. Therefore, DIDM should be considered in every case of newly diagnosed diabetes in patients treated with diabetogenic medications. Epidemiology studies of DIDM in children mainly focus on post-transplant diabetes mellitus, which refers to a specific subgroup of patients. Referring to the Canadian National Surveillance study, we can estimate that the DIDM is less frequent in the general paediatric population than the diabetes type 2 (DM2) population [1].
Drug-induced diabetes mellitus can be caused by mechanisms leading to decreased insulin secretion (decreased synthesis, impaired signalling, destruction of β-cells), increased peripheral insulin sensitivity, or increased glucose influx. Many drugs probably disturb more than one pathway of glucose homeostasis, but one of them is usually dominant. Although not every patient treated with diabetogenic drugs will develop DIDM, some of them will develop pre-diabetes states (impaired fasting glucose or impaired glucose tolerance), while others will stay euglycemic. The development and severity of glucose metabolism derangement are related to host-specific factors, such as obesity, insulin resistance (IR), quantity and condition of islet cells, family history of DM2, genetic predisposition, and environmental influences. The diabetogenicity of most medications is dose dependent. Combining 2 or more diabetogenic drugs also intensifies glucose metabolism impairment. Moreover, long-term therapy with certain medications can lead to obesity, a risk factor for DM2, and pre-diabetes states [2–4]. More detailed data about the drug-related risk of diabetes in patients treated for primary proteinuric kidney diseases have been published. Research performed on a group of adults and children showed that the patients on steroid treatment have a higher risk for diabetes onset than patients not exposed to steroids. In addition, the use of calcineurin inhibitors also increases the risk of diabetes [5]. The persistency of DIDM is not simple to evaluate. Most of the evidence is anecdotal. However, it is generally considered that if the treatment does not damage the pancreatic β-cells in an irreversible way, the severity of glucose homeostasis impairment should be reduced partially (to pre-diabetes state) or totally (to euglycaemia) after the drug discontinuation. Research performed on a small group showed that the pre-diabetes states persisted in 71% of children with nephrotic syndrome (NS) treated with steroids and calcineurin inhibitors (CNIs) [6]. The series of case reports on a similar group indicates only a transient glucose metabolism disturbance [7].
Drug-induced diabetes mellitus is often treated with insulin injections due to insufficient evidence about the pathway-focused therapy and a lack of legal approval to use most oral antidiabetics in children.
Here we report a case of diabetes diagnosed 2 months after the introduction of tacrolimus (Tac) to the NS therapy. The parents of the patient gave their informed consent to use the medical records of their son for scientific purposes.
Case presentation
A 13-year-old Caucasian male with a 10-year history of submicroscopic nephrotic syndrome was admitted to the Department of Nephrology for a check-up 2 months after the introduction of Tac treatment. His medical history also included non-inflammatory hypothyroidism, hypertension, dyslipidaemia, and familial risk of DM2 (both parents, maternal grandmother, sister of his mother).
For the preceding 4 years, he had stayed over the 95th percentile of body mass index, which means obesity, and in the 25th percentile of height (data assessed based on Polish growth references [8]). He had not shown a significant weight loss in the preceding 12 months.
Steroid sparing agents were introduced to the NS therapy early in the course of the disease because of steroid dependency and frequent relapses. In the past, he had been treated with cyclosporine A (CsA) and mycophenolate mofetil (MMF), but they did not improve the length of remission periods more than the standard prednisone treatment. Enalapril had been introduced for proteinuria reduction, and it was changed to amlodipine when our patient developed systolic hypertension.
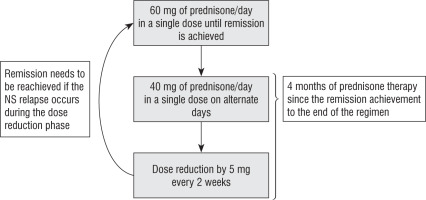
During the hospitalization our patient was in the second month of a 4-month course of prednisone therapy. The regimen of steroid treatment is shown in Figure 1. He was also treated with 2 mg of tacrolimus twice a day and 75 µg of L-thyroxine.
The hypothyroidism was treated with L-thyroxine. The dose was systematically corrected depending on the blood level of thyroid-stimulating hormone. In addition, our patient was supplemented with vitamin D, calcium, magnesium, and potassium.
On admission, he complained of dry mouth, but he denied polyuria and polydipsia. In the physical examination, he showed a Cushingoid appearance (pink stretch marks and characteristic fat tissue distribution). His weight was 68.4 kg (94th percentile), and his height was 153 cm (26th percentile). The body mass index (BMI) calculated using these data was 29.2 kg/m2 (98th percentile), which corresponds to obesity.
Blood tests showed high postprandial (364 mg/dl) and fasting (143 mg/dl) plasma glucose levels. The glycated A1c fraction of haemoglobin (HbA1c) was 10.2%. There was no ketoacidosis or electrolytes disturbances, except for a low magnesium level (1.41 mg/dl; normal range 1.7–2.55 mg/dl). Parameters of thyroid function indicated euthyreosis. The concentration of Tac was above the recommended level (18.5 ng/ml, recommended level 4–8 ng/ml). Urinalysis showed only glucosuria. Following the guidelines published by the ISPAD [11], the fasting plasma glucose was re-tested the next day, and the result was 154 mg/dl, which fulfilled the diagnosis criteria for diabetes (2 abnormal readings ≥ 126 mg/dl).
The patient was transferred to the Department of Diabetology to identify the type of diabetes and choose a complementary treatment. A broad spectrum of laboratory analyses was performed (Table I).
Table I
Laboratory test results
At first, he was diagnosed with an unclassified type of diabetes mellitus [12]. After obtaining β-cell autoantibody results and C-peptide levels (after 3 weeks), diabetes mellitus type 1 (DM1) was excluded. Therefore, DM2 and DIDM were the 2 most probable causes, but the presented symptoms and results of laboratory analyses did not allow us to confirm any one of them.
Because of the high blood concentration of Tac, the daily dose was reduced from 4 mg to 2 mg (1 mg twice a day). Intensive insulin therapy was initiated because prednisone-induced diabetes was suspected [13, 14], and the HbA1c was greater than 8.5% [15]. The patient was discharged home after completing training with a diabetic educator. The initial total daily dose of insulin (TDD) was 21 units (0.31 U/kg; 3 units of basal, and 18 units of meal boluses).
The steroid withdrawal was completed 2 months after the diabetes diagnosis. Then, the HbA1c was 6.5%, and the average TDD had not changed. A month after the prednisone withdrawal, the average TDD and TDD per kilogram were constantly unchanged. HbA1c was reduced to 5.5% and remained at this level (±0.1%) for a year; during that year, he reported occasional hyperglycaemia (up to 250 mg/dl) after high glycaemic index meals. He denied hypoglycaemia, which was confirmed by the glucometer measurement history. The low TDD/kg and HbA1c comparable to the healthy individuals was accompanied by sporadic hyperglycaemia, which confirmed that the diabetes was still present, despite the steroid withdrawal.
The fast improvement of HbA1c prompted us to consider insulin withdrawal gradually with the introduction of oral antidiabetic agents. Unfortunately, without the DIDM exclusion, metformin (the only oral antidiabetic agent in Poland that is approved for paediatric patients) could be introduced only off-label. We decided not to change the treatment because of the small amount of evidence about metformin usage in children with DIDM. Our patient stayed on insulin until the exclusion of DIDM was possible.
An attempt to discontinue Tac after 2 years of treatment caused a recurrence of proteinuria. Remission could not be achieved with the use of orally administered prednisone (Figure 1). It was achieved by 6 pulses of methylprednisolone. MMF was introduced to prevent relapses of NS. Interestingly, we did not observe diabetes metabolic control deterioration when the steroids were re-introduced, and the insulin doses were persistently low. If the steroid withdrawal ends in remission that can be sustained using MMF, we will try to re-evaluate the pathway responsible for the diabetes in our patient.
Review of the literature
Diabetes in our patient was diagnosed accidentally. He was admitted to the hospital for a completely different reason. Interestingly, he was oligosymptomatic, which is atypical in the clinical picture of diabetes in children. Furthermore, diabetes in our patient was also atypical. It looked to be the outcome of pathways involved in the pathogenesis of DM2 and DIDM. We think that we are the first to present a case report of a child with such a specific subtype of diabetes. The large heterogenicity means that DIDM should be discussed more likely as a complication of treatment using a specific drug than the one heterogenic type of diabetes. Within this publication, we want to focus on DIDM in paediatric patients treated for NS, although we will partially discuss some general aspects of DIDM.
Drug-induced diabetes mellitus is omitted in the guidelines published by the leading nephrology associations, despite the fact that the widely prescribed prednisone and CNIs are diabetogenic. Moreover, steroids – the first-line treatment in NS [9] – are also mentioned as the most common cause of iatrogenic hyperglycaemia [3].
The main pathways of steroid-induced hyperglycaemia are the increment of IR and the intensification of hepatic gluconeogenesis. They mainly cause postprandial rather than fasting hyperglycaemia. Moreover, long-term steroid treatment leads to weight gain, which increases IR. We should keep in mind that they could cause diabetes directly – then it is called glucocorticoid-induced diabetes mellitus (GIDM), or indirectly – DM2 caused by steroid-induced overweight or obesity [2]. Interestingly, the increment of IR and risk of DM2 are dose dependent [16, 17].
The pathophysiology of DIDM caused by the CNIs requires more research to establish a consensus in the future. Most publications about CNIs diabetogenicity are based on experimental studies, which cannot be fully translated into the human body. On the other hand, the clinical studies mainly relate to new-onset diabetes after transplantation (NODAT). Pharmacotherapy after solid organ transplantation commonly consists of diabetogenic steroids, which could cause biased results. It is also worth mentioning that the NODAT has a wider pathogenesis than the diabetogenic action of drugs and should be distinguished from DIDM [18].
The impaired insulin secretion is the most frequently indicated pathway of DIDM caused by CNIs. The changes in β-cells induced by CNIs seem to be reversible after treatment discontinuation. The diabetogenicity of CNIs is dose dependent. Tac is considered to be more diabetogenic than CsA. There is some experimental evidence that the CNIs could increase the IR, which is inconsistent with the results of clinical research [2, 17, 19]. Some studies on solid organ transplant recipients revealed that the Tac could decrease serum magnesium levels. Hypomagnesaemia is considered to be a factor that decreases insulin secretion and intensifies the IR. It is also described as a risk factor of NODAT [18, 20–22].
The main problem is the lack of a widely accepted definition and diagnostic criteria for DIDM. It means that all of the patients treated with diabetogenic medications should be classified as DIDM until the DIDM can be excluded. Because of that, our patient described in the case report above could not be recognized as DM2, despite the comorbidities characteristic for metabolic syndrome (obesity, hypertension, dyslipidaemia) and familial history of DM2.
Furthermore, we need screening recommendations that will be adapted to the specific disease and used medications. The lack of these recommendations causes delayed diagnosis of DIDM. Widely approved screening intervals (every 3 years, or yearly in some cases) in the groups with a higher risk of diabetes are not adapted to the characteristics of DIDM [13, 23]. The patients should be additionally screened at crucial moments of treatment; for example, when the pharmacotherapy is modified. Regarding the reported case, we can notice the coincidence of prednisone withdrawal, tacrolimus introduction, and diabetes diagnosis. That coincidence complicated the differential diagnosis between DIDM and DM2. If the screening had been done before the introduction of Tac, more of the data could have been analysed before introducing new medication. In the presence of diabetes or pre-diabetes, the non-diabetogenic steroid-sparing agent would be introduced instead of Tac. On the other hand, the euglycaemic state preceding the introduction of new treatment would indicate that the diabetes was caused by Tac or by a combination of diabetogenic effects of Tac and prednisone.
Our position on the diagnostic process of DIDM in paediatric patients is consistent with the position of Tosur et al. presented in the publication “Medication-induced hyperglycaemia: Paediatric perspective” [2]. In our opinion, patients with suspected DIDM should undergo a fasting glucose test. If fasting glucose is in the normal range, the oral glucose tolerance test (OGTT) should be started immediately to verify both fasting and postprandial glycaemia. The OGTT should not be neglected, because evidence from studies on DM2 in adults shows that not performing an OGTT could lead to underdiagnosis of diabetes [24]. Furthermore, a study on children treated with steroids and CNIs because of the refractory NS showed that 40% of all glucose homeostasis disturbances were diagnosed only by the 2-hour OGTT [6].
HbA1c may be an additional laboratory test, but it may be helpful only in cases of chronic treatment because at least 3 months of dysglycaemia are required to influence the level of HbA1c significantly [25]. The long-term monitoring of the relative increase in the maximum level of HbA1c (RIM-HbA1c) is an interesting option. RIM-HbA1c is defined as the difference between the maximum level of HbA1c during the prescription period and the pre-prescription level of HbA1c. It was investigated by Imatoh et al. in the context of GIDM [26], although the RIM-HbA1c should be investigated further to evaluate and standardize the method for DIDM detection.
We realize that the oral glucose tolerance test is challenging to implement for routine diagnostics repeated in short intervals (monthly or every 3 months). Therefore, periodic diagnostics should include HbA1c and fasting glucose tests, and the OGTT should be repeated in long intervals (i.e. yearly) or when diabetes is suspected based on clinical signs and symptoms. A daily glucose profile made periodically using a glucometer or continuous glucose monitoring could provide a wealth of information about fasting and postprandial glucose level. However, all these methods should be checked in clinical studies to verify which is the most effective in detecting DIDM in children. Probably a variety of methods will be effective depending on the pathogenesis of DIDM.
The fasting and postprandial (or glucagon-stimulated) C-peptide and pancreatic islet autoantibodies are obligatorily checked in all newly recognized diabetic patients in our clinic, and we think this is good practice to assess the underlying pathomechanism of hyperglycaemia. The determination of the C-peptide level has some disadvantages. The cause of low C-peptide may be problematic to distinguish because it may be the result of β-cell destruction (which must be treated with insulin) or disruption of the mechanism of insulin secretion from β-cells (which may be treated with non-insulin medications in some cases – it is further described later in the discussion). Pancreatic islet autoantibodies may be helpful to distinguish the autoimmune background of β-cells destruction. However, undetectable autoantibodies may indicate DIDM, maturity onset diabetes of the young (MODY), or autoantibody-negative DM1 [27, 28]. In the cases with low C-peptide and negative antibodies, it could be difficult to make a final diagnosis. In such a variant, the discontinuation of diabetogenic medication seems to be an optimal way of finally differentiating the type of diabetes.
In conclusion, the diagnostic process of DIDM is composed of many stages of laboratory tests aimed at broadening the information about the pathophysiology of diabetes. Because DIDM could imitate other types of diabetes, and nowadays, it is difficult to point to a factor that could indicate DIDM. Evidence-based medicine (EBM) gives us some information about pathways of glucose homeostasis, which are disrupted by the action of certain drugs. This knowledge is beneficial when it is compared with the clinical image of patients with suspected DIDM.
Treatment of DIDM in the paediatric population is another branch of knowledge based on anecdotal evidence. Undeniably, the easiest way to treat DIDM is to withdraw diabetogenic medication, but in some cases it is not possible. Physical activity and maintaining an appropriate diet – the non-pharmacological treatment basis – is probably the appropriate approach in DIDM, as with other types of diabetes.
The pharmacological treatment of DIDM is based on subcutaneous insulin injections, regardless of the underlying pathology. Undeniably, insulin is an effective, widely used medication, which may be used to treat all types of diabetes. Unfortunately, insulin is associated with a very high risk of hypoglycaemia, a frequent and potentially fatal side effect of insulin. It cannot be forgotten that hypoglycaemia can cause anxiety to patients and their parents, which worsens the life quality of whole families [29].
Hyperinsulinaemia is another problem that is still ignored and not a thoroughly researched phenomenon. Usually, the states with deficiency or excess of various hormones are considered as pathologic and badly affecting the physiological development of a child. So maybe we should look at the insulin treatment through the prism of iatrogenic hyperinsulinaemia.
Hyperinsulinaemia leads to weight gain, and if it is applied to treat DIDM caused by other medication leading to weight gain, it increases the risk of overweight and obesity, which is linked to IR and DM2 [4, 30]. Some evidence indicates that the iatrogenic hyperinsulinaemia caused by non-physiological delivery of insulin drives IR in patients with DM1 [31]. Patients with DM2 treated with insulin also had higher IR than the control group of DM2 patients treated with non-insulin agents [32]. If a similar mechanism exists in DIDM, the insulin treatment may increase the risk of persistency of the DIDM or transition to DM2. Quoted arguments can lead us to the hypothesis that insulin should not be used as the first-line medication to treat all cases of DIDM in the paediatric population. If our patients are hyperglycaemic despite their ability to produce insulin in physiological concentrations, it may be a good idea to ask ourselves why their own insulin acts ineffectively, and if we could improve its action by using other medications.
When we analyse the main pathways of DIDM, it seems logical to use insulin in cases of low C-peptide DIDM (i.e. after the destruction of islet cells) and non-insulin antidiabetics in cases with normal or high C-peptide, where the hyperglycaemia is caused mainly by the IR. Therefore, in our opinion, if the DIDM is not caused by function loss of the β-cells, the pharmacotherapy might be introduced in a similar algorithm as in the DM2 [15]. If oral antidiabetics are insufficient to achieve treatment targets, or if the β-cell function is disturbed, insulin should be introduced. However, in the times of EBM, we cannot rely on smart-sounding ideas, we need non-biased evidence based on properly conducted research.
As mentioned above, steroids and CNIs are the leading causes of DIDM in the course of NS. The usage of oral antidiabetics in GIDM is still unclear, especially in children. Interestingly, the Joint British Diabetes Societies for Impatient Care (JBDS-IP) published guidelines in 2014 in which GIDM and the use of steroids in DM2 are widely discussed. JBDS-IP recommends using insulin as the most effective agent to achieve glycaemic control in cases of steroid-induced diabetes, although they also point out that gliclazide or pioglitazone monotherapy may also be effective [33]. Unfortunately, these drugs are not yet approved for the treatment of children. Another oral antidiabetic agent, metformin, is potentially effective and safe to treat GIDM in children, and it has also been tested and approved for use in the paediatric population [34].
There is limited evidence of treatment possibilities of DIDM caused by CNIs.
It seems that the conversion from Tac to CsA is the easiest option to reverse the DIDM [17]. As we mentioned before, Tac could cause hypomagnesaemia. Therefore, supplementation of magnesium could be beneficial. Interestingly, the use of exendin-4, which is an agonist of the glucagon-like peptide-1 receptor (GLP-1RA), prevented the Tac-induced dysfunction of islet cells [19, 35].
The complications begin when the patients are treated with more than one diabetogenic medication, which is not uncommon in NS patients. Perhaps liraglutide – a GLP-1R agonist approved by the United States Food and Drug Administration to treat DM2 in children – can be used more widely in the future in cases of DIDM caused by NS treatment [2]. Theoretically, it should be an excellent choice to prevent the postprandial hyperglycaemia caused by steroids and Tac-induced dysfunction of islet cells. Moreover, the results of retrospective study made by Thangavelu et al. showed that liraglutide was as effective in NODAT patients as in DM2 patients [36]. Liraglutide could probably be used in unclear cases like the one reported by us. It should be investigated more widely to know more about the effectiveness of such therapy.
It is good to remember that the development of newer, more effective drugs to treat nephrotic syndrome has saved many lives. Before the first pharmacotherapy attempts, the mortality of NS was 40–65%, whereas nowadays it is lower than 1% [9, 37]. With such results, we need to go one step further and detect and minimize the side effects of therapy.
A multitude of treatment options combined with appropriate therapy regimens allows us to reduce the side effects of used medications. Unfortunately, nowadays, we cannot fully predict which patients have a high risk of developing side effects. In the future, pharmacogenetics and pharmacogenomics should improve the selection of effective personalized therapy with minimalized risk of side effects.
In our opinion, future investigations should be directed towards personalized medicine, using all the advantages of medications and their pleiotropic actions. This approach could be observed in the metformin treatment of DIDM in patients with acute lymphoblastic leukaemia [34]. In NS patients, the introduction of dipeptidyl peptidase-4 inhibitors may be beneficial [38, 39].
Our publication emphasizes the overlooked problem of DIDM in children. We wanted to summarize the possibilities of diagnosing and treating DIDM through the complex discussion section. Our publication cannot be used as a recommendation. Its purpose is to remind our readers about DIDM and indicate the possibilities to conduct further research, leading to the development of guidelines in the future.
Conclusions
Drug-induced diabetes mellitus is not one subtype of diabetes; it is a heterogenic group of glycaemic disturbances caused by medications. It should be subgrouped by the mechanism or by the drug group. There is a great need to perform more research on DIDM in paediatric patients because a large part of the knowledge is based on the pathophysiology and experimental research.
All newly diagnosed diabetic patients treated with diabetogenic medications should undergo an extensive diagnostic process to exclude or confirm DIDM. Periodic screening for diabetes should be performed on all patients treated with diabetogenic medications.
Insulin remains the primary medication to treat DIDM in the paediatric population. However, it is primarily chosen due to the lack of randomized controlled studies on the efficacy and safety of non-insulin antidiabetics for DIDM treatment in children. Non-insulin antidiabetics have a lower risk of hypoglycaemia and weight gain. Additionally, they do not lead to iatrogenic hyperinsulinaemia. Unfortunately, only a few of them are approved for use in children, and only in the case of DM2.
The new treatment methods for DIDM should focus on the underlying cause of glycaemic disturbances.

 POLSKI
POLSKI