Introduction
Diabetes mellitus (DM) is by definition a metabolic disorder whose primary feature is chronic hyperglycemia, which can be attributed to disrupted insulin secretion, disturbed insulin effect or lack of insulin [1]. In type 1, which belongs to autoimmunological disorders and accounts for about 10% of DM, the immune system destroys beta cells of pancreas, what leads to insufficient insulin production [1]. Diabetes mellitus type 1 is one of the most common metabolic disorders affecting pediatric population. SEARCH reported that in the United States, in 2009, an estimated 167,000 youth lived with type 1 diabetes. The overall prevalence (cases/1,000) was 1.93. It was similar in boys and girls and increased with age from 0.82 in children age 0–9 years to 2.97 in youth age 10–19 years [2].
Currently the foundation of insulin therapy for DM1 is a combination of basal insulin and bolus insulin associated with nutrient consumption and daily activities [1]. Nowadays, it is common to provide continuous subcutaneous insulin infusion (CSII) among pediatric patients suffering from DM1. Studies show that it allows to reduce both the level of glycated hemoglobin levels and frequency of severe hypoglycemia. Moreover, application of continuous glucose monitoring systems (CGM) is a worth-mentioning method of improvement of the glycemic control [3]. Rational treatment of DM is really important as any imbalances can cause hiper- or hypoglycemia, which can lead to hypoglycemic encephalopathy (HE).
HE is a type of encephalopathy resulting from extremely low blood glucose level. Symptoms are not specific and can be misdiagnosed very often [4]. It can occur during deep and/or prolonged hypoglycemia, which may be a result of inadequately controlled diabetes. HE is generally caused by irrational use of pharmacologic agents, insufficient food intake, endogenous insulin secretion, liver and kidney disorders, alcoholism, and various endocrine diseases. When glucose level is lower than 2.3 mmol/l, the patient may fall into a coma [1]. Identification of a hypoglycemic patient is critical due to potential adverse effects, including aforementioned coma and/or death. In literature there are many cases presenting that encephalopathy, in general, can lead to epilepsy [5]. However, as far as our knowledge is concerned, there is lack of information about hypoglycemic encephalopathy leading to drug resistant epilepsy. Moreover, there are very few cases with such a rapid course and serious consequences as the one presented in our work.
Here, we report a case of hypoglycemic encephalopathy as the consequences of a metabolic dysfunction. Furthermore, incorrect control of blood glucose in this case was the causative factor of drug resistant epilepsy. Informed consent of caregiver was taken.
Case report
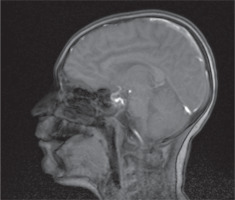
An 11-year-old male patient, without perinatal abnormalities, properly developing, diagnosed at age of 4 with type 1 diabetes mellitus treated with the use of insulin pump was admitted to the Pediatric Neurology Department because of multiple incidents of seizures. The history of his antidiabetic treatment showed lack of control of the disease, which led to chronic hyperglycemia (at admission HbA1c % was 8.1%) throughout the whole duration time of diabetes, despite gradual increasing doses of insulin. Thus, his mother was instructed to increase mealtime insulin dose. During the last appointment in the day-care diabetic unit before the hospitalization the total daily insulin dose was calculated and it was 48 units per day (1.2 unit/kg/day). Due to recuring incidents of symptomless hypoglycemia, measured with glucometer, mostly at night and after physical activity, CGM system was provided. Afterwards, despite of multiple hypoglycemia (up to 20 mg/dl) alarms parents did not adjust the insulin intake, which led to severe hypoglycemia and boy was found unconscious by his mother. The blood glucose level on the glucometer was 35 mg/dl. The last recorded value by CGM was 65 mg/dl. Due to presenting severe seizures and trismus oral glucose intake was not possible, so patient’s mother called the ambulance. He was given intravenous glucose by the ambulance service without recovering consciousness or other improvement of patient’s state. The patient was assessed to have 5 points in the Glasgow Coma Scale, and subsequently was intubated and given an intravenous infusion of glucose and midazolam without any improvement. The boy was admitted to the Intensive Care Unit. Laboratory examination of cerebrospinal fluid did not reveal any features of infection or inflammation. Head CT and chest CT were performed and revealed cerebral edema, hypo-dense areas and obliteration of temporal lobes of the brain as well as inflammatory features in the left lung. He was provided with mannitol, diuretics, broad-spectrum antibiotics (tazobactam and piperacillin), benzodiazepines and parenteral nutrition. The patient was treated in the ICU for 4 days, after which he was extubated and transferred to the Diabetology Clinic. CSF autoantibodies tests were performed and due to the similarity of clinical symptoms to autoimmune encephalitis a 5-days intravenous immunoglobulin treatment was administered. However, results of autoimmunobodies tests did not confirm autoimmune encephalitis. Go Brain MRI was performed and showed in both cerebral hemispheres, symmetrically, elevated white matter signal, mainly in the subcortex and cortex of the frontal and occipital and parietal lobes with features of diffusion restriction. Similar changes were seen in the posterior branches of the internal capsule (Fig. 1). The whole image indicated hypoglycemic encephalopathy without features of cerebral edema. EEG revealed generalized slow brain activity, without obvious epileptiform. Due to severe neurological condition he was transferred to the Pediatric Neurology Department. The patient was conscious but without logical contact. He was in constant psychomotor restlessness, did not follow commands and did not cooperate during the examination. Control brain MRI was performed and showed regression of previously described features. After stabilization of the patients state he was discharged. In the next two months, due to the brain damage caused by a hypoglycemic episode the patient developed epileptic seizures. After the first seizure with the morphology of a generalized convulsion he was transported to the hospital. Infection, dehydration, hypoglycemia or other causes that could have led to seizures were excluded. Since then the patient has 1–2 seizures per day presenting with atonia, right upper limb clonus and clonus of lower limb, which resolve spontaneously. Once every few months he has a generalized tonic-clonic seizure. Since the beginning of the epileptic seizures, antiepileptic treatment was induced. He was provided with a variety of antiepileptic drugs, such as carbamazepine, oxcarbazepine, valproic acid, lamotriginum, primidonum, lacosamidum, clobasamum, vigabatrinum and sultiamum. Currently, he is treated with clobasamum, sultiamum and lacosamidum. Unfortunately, none of the aforementioned drugs yielded with satisfactory results so far and the patient is still suffering from drug-resistant epilepsy. The patient is under regular control of day care diabetic unit. The basal insulin dose was decreased and mother adequately adjusts insulin dose according to the glycemia level. That allowed to obtain satisfactory control of glycemia and lack of severe incidents of hypoglycemia. Nonetheless, such serious course of hypoglycemia significantly affected patient’s general state and development. Before this serious event he was properly developing and functioning boy and currently he is intellectually disabled to a great extent.
Discussion
There is no pathognomonic symptomatology for the HE and literature discussing pediatric patients dealing with this disorder is rather poor. Nevertheless, HE has some overlapping symptoms with neonatal hypoglycemia, such as: irritability, tremor and seizures. It is known that epilepsy with seizures is one of the most serious consequences of hypoglycemia in pediatric population [6]. According to the literature, seizures have been described as focal jerking of the arms and legs, tonic or tonic-clonic [6]. However, not a single scientific paper reporting about drug-resistant epilepsy induced by HE has been found.
It is important to acknowledge the importance of MRI imagining, while dealing HE. MRI reveals diffuse abnormal intensity in cortex and basal ganglia region. Extensive lesions observed in MRI scanning may predict serious neurological outcomes. Moreover, the degree of lesions may also affect the degree of presenting neurological symptoms [7].
In our patient’s case lesions were observed in occipital and parietal lobes of the brain, which is a typical localization of the MRI findings among patients with HE, as these regions are relatively more sensitive to hypoglycemia [7].
When it comes to the possible explanation of the correlation between glucose level and epilepsy development, glucose is a crucial element for the brain function. Its metabolism is the source of precursors and energy for biosynthesis of several neurotransmitters. Neuronal computation, information processing, and maintaining of ion gradients across neuronal membranes consume the biggest portion of brain energy [8]. Glucose homeostasis is based on the group of specialized glucose sensing neurons. It is known that some of the brain regions are selectively vulnerable to hypoglycemia including the second and the third superficial layers of the cerebral cortex, the dentate gyrus, the subiculum, the CA1 regions in the hippocampus, and the caudate-putamen nuclei [9]. Several mechanisms of neuronal damage in the course of hypoglycemia are discussed. One of them is the extensive activation of poly ADP-ribose polymerase-1 due to increased release of zinc as the result of neuronal depolarization induced by low level of glucose. Extensive release of zinc may also affect activity of many receptors including those for NMDA, GABA-A, ATP, and glycine as well as voltage-gated Na+ and Ca2+ channels, what has been associated with the promotion of neuronal death [10]. Another mechanism is the elevation of brain extracellular glutamate concentration, which leads to neuronal death. Moreover, increased glutamate level may lead to accumulation of aspartate, which may also activate glutamate receptors and contribute to excitotoxicity [8]. Ultimately, all of the above mechanisms could lead to brain injury and finally lead do the development of the drug-resistant epilepsy and HE [10].
A thorough understanding of these mechanisms will lead to better insight into the pathophysiology of multiple diverse disorders of the brain and allow the development of novel treatment strategies. A large variety of central nervous system pathologies such as Parkinson’s disease or Alzheimer’s disease may be the consequence of disturbed glucose energy metabolism [8].
Conclusions
Dealing with patients presenting seizures we always have to take metabolic diseases into account, especially among diabetic patients. Such patients require multidisciplinar approach and high attention. Glucose is one of the key metabolic agents for the proper brain function and any imbalances in its blood level may impair the neuronal computation. Thus, adequate glycemia control is crucial to avoid any disturbances, as they may lead to severe consequences, such as HE and drug-resistant epilepsy.

 ENGLISH
ENGLISH