Introduction
Understanding the basis of phenomena occurring in the human body is a key element in the diagnosis and treatment of diseases, as well as their prevention. Preeclampsia - also known as EPH-gestosis - is a complex set of symptoms occurring during pregnancy. It is a condition in which the well-being of both the pregnant woman and the fetus is at risk. If preeclampsia occurs during pregnancy, there is an increased risk of developing cardiovascular diseases later in the life of the woman and her child. Risk factors for preeclampsia include cardiovascular diseases, autoimmune diseases, kidney diseases, diabetes, as well as obesity and long intervals between pregnancies. There are three classifications of preeclampsia - early and late, maternal and placental, and severe and mild forms[1].
The criteria for diagnosing preeclampsia include hypertension diagnosed after 20 hbd and proteinuria (>0.3 g per day). Common abnormalities in laboratory tests include: thrombocytopenia, high creatinine concentration, increased activity of liver transaminases, and disturbances in the parameters of the coagulation system. The clinical onset of preeclampsia is ambiguous and may vary. Symptoms of EPH-gestosis in pregnancy include abdominal pain, most often in the upper right quadrant, hemolysis, neurological symptoms in the form of: headaches, seeing spots, visual disturbances, mental disorders; pulmonary edema, cerebral edema. They are accompanied by symptoms from the developing fetus. These include fetal intrauterine growth restriction (IUGR), abnormal flow in the umbilical artery and even intrauterine fetal death [1,2].
Early preeclampsia is diagnosed before 34 hbd and affects approximately 20% of cases. Its pathogenesis is abnormal invasion of the trophoblast into the uterine spiral arteries, which is related to improper blood flow within the placenta. Preeclampsia of this type may cause numerous complications related to both the fetal life of the child and the course of pregnancy, including: intrauterine fetal hypotrophy, CNS hypoxia, perinatal death, too few blood cells, premature birth, respiratory disorders and diseases later in life, i.e. cardiovascular diseases (arterial hypertension, atherosclerosis) and diabetes [1,3,4,5].
Late-onset preeclampsia occurs in over 80% of cases after the 34th week of pregnancy. Its pathomechanism is related to diseases occurring in pregnant women during the preconception period. These include kidney and heart diseases, obesity and diabetes. In this form, the function of the uterine spiral arteries is preserved [1,3,4].
Pathogenesis
Pregnancy is a period of numerous changes both in the developing fetus and the mother’s body as a whole. This condition is influenced by substances secreted by the placenta. The pathogenesis of preeclampsia is not fully known. Many researchers point out its complex nature, taking into account both maternal and fetal risk factors. It is indicated that it is related to increased secretion of anti-angiogenic proteins by the placenta, and the genetic basis of this condition is increasingly attracting attention. It is estimated that over 1/3 of preeclampsia cases are related to genetic conditions. This thesis is confirmed not only by the latest molecular studies [6,7], but also by differences. Based on them, it can be concluded that Caucasian women experience preeclampsia less often than pregnant black women, which is undoubtedly related to racial differences in the incidence of hypertension[1,6,8]. Numerous scientific reports present different ideas about which gene has the greatest impact on the occurrence of this disease. Most authors agree with the idea that polymorphism in many genes is responsible for this condition[8,9].
Objective of the work
The aim of the study was to present typical deviations from reference values in women with symptoms of preeclampsia and compare them with the results of healthy pregnant women, which may allow for noticing differences in earlier stages of pregnancy rather than the appearance of clinical symptoms. This analysis aims to determine whether it is justified to conduct further statistical research within the studied population in terms of the selected statistical methods and the analyzed variables.
Material and methods
The study included patients hospitalized at the Department of Gynecology and Obstetrics. The preliminary analysis was based on the test results of 75 patients. Three patients were excluded from the study due to incomplete data for comparative analysis. Ultimately, the analysis was based on the test results of 72 patients.
The statistical analysis was performed using the Statistica 13 computer program. The obtained results were subjected to the Chi2 test, called the Pearson test. Cramer’s V coefficients and Spearman’s rank correlation were calculated.
The Bioethics Committee of the Collegium Medicum of the Jan Kochanowski University has consented to the study under no. 64/2021. The information necessary for the study was obtained in accordance with the personal data protection policy.
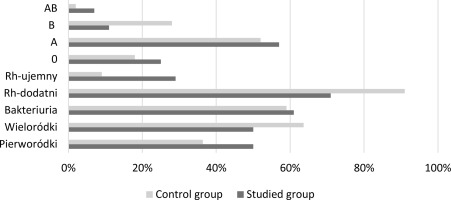
The study included women of reproductive age, both primiparous and multiparous women in the third trimester of pregnancy. Among the examined patients, 28 patients constituted the research group - patients with preeclampsia, 44 patients constituted the control group. The control group included healthy pregnant women with normal blood pressure and no comorbidities. This was intended to obtain the most reliable comparison of individual groups. The research group consisted of patients diagnosed with preeclampsia. The pressure values obtained during the examination exceeded the upper limit of the norm: Above 140 mmHg for systolic pressure and 90 mmHg for diastolic pressure, proteinuria was observed in this group - above 0.3g of protein. In the research group, 14 patients were primiparas. The remaining pregnant women were multiparous. In the case of the control group, 16 pregnant women were pregnant for the first time, 28 were multiparous.
In both study groups, bacteriuria was detected in the general urine test, and their percentage share in the population was similar. Both groups had patients with Rh-positive and Rh-negative blood. It should be emphasized that in the case of women with preeclampsia, the percentage of Rh-negative pregnant women was much higher. In terms of the main groups (AB0), the studied population included patients with every possible blood group variant, but it is important that the share of individual possible variants was different. Particularly significant differences are visible for groups AB and B.
Table 1
Relationship between individual urine sediment test parameters and preeclampsia
| x2 | Df | p | V | ρ | |
|---|---|---|---|---|---|
| Red blood cells in the urine | 2,11 | 1 | 0,146 | 0,17 | 0,17 |
| Leukocytes in urine | 0,90 | 1 | 0,34 | 0,11 | 0,11 |
| Bacteriuria | 0,18 | 1 | 0,89 | 0,16 | 0,16 |
Based on the statistical calculations performed, it can be concluded that urine sediment parameters, i.e. the presence of erythrocytes, the presence of leukocytes and bacteriuria occur with a similar frequency in the general population of pregnant patients. Therefore, they have no predictive value.
Table 2
The relationship between individual blood parameters and preeclampsia
| x2 | Df | p | V | ρ | |
|---|---|---|---|---|---|
| Rh factor | 4,67 | 1 | <0,05 | 0,25 | 0,25 |
| Blood type | 3,68 | 3 | 0,29 | 0,22 | 0,22 |
| Anemia | 37,0 | 4 | <0,05 | 1 | 0,85 |
The analysis of statistical data revealed a significant correlation between the Rh factor and the occurrence of pre-eclampsia. It was noticed that among patients with preeclampsia, Rh-negative people were more common than among healthy women. No correlations were observed within the main groups. The hemoglobin value classified in the categories of normal result/anemia can be considered statistically significant. Based on the data obtained, a higher percentage of patients with anemia was observed in the research group than in the control group.
Table 3
The relationship between liver enzymes and preeclampsia
| x2 | Df | p | V | P | |
|---|---|---|---|---|---|
| AST | 1,19 | 1 | 0,27 | 0,12 | 0,12 |
| ALT | 1,25 | 3 | 0,26 | 0,13 | 0,13 |
In the study group, statistical analysis showed that there are no significant correlations between the activity of liver enzymes and the occurrence of pre-eclampsia. Moreover, it should be emphasized that in the study population there are no patients with abnormal results in the assessment of the coagulation system, i.e. APTT, PT, INR, D-dimer. These parameters are within the range of reference values for pregnant women in the study population.
Discussion
The activity of liver enzymes - aminotransferases: alanine and aspartate - is a parameter aimed at monitoring the functioning of the liver and bile ducts. A controversial impact of changes occurring during pregnancy on these enzymes has been noted, as sources provide different data regarding their increase or remaining within normal limits. Analyzing the data contained in scientific articles, a slight increase in transaminase activity can be noticed in the third trimester of pregnancy. Most sources indicate that the reference values for pregnant women in terms of ASPAT and ALAT should not be changed compared to the general population. Therefore, any increase in transaminase activity should be the basis for expanding the diagnostics [10]. In the study, it seemed important to check whether the activity of liver enzymes was statistically different in the study groups. The analyzes allowed us to clearly state that there is no significant relationship between the activity of aminotransferases and the occurrence of preeclampsia. Increased liver parameters are more often observed in women with EPH-gestosis, but this value is not statistically significant. The study by Greiner et al. substantiates that in patients with full-blown preeclampsia, LFT values above threshold are not independently associated with an increased risk of adverse maternal or neonatal outcomes. This study also mentioned that only a small percentage of patients (13.8%) had liver parameters above the reference values [11].
Blood group determination is a routine test during pregnancy. Its main aim is to determine the Rh factor and plan possible actions to prevent serological conflict. Moreover, knowledge of the blood type is important if a blood transfusion is necessary. Life-threatening situations require immediate therapeutic decisions, which is of great importance during pregnancy due to the well-being of the developing fetus. Over the years, the methods of determining blood groups have undergone enormous progress, which undoubtedly translates into the comfort of treatment and the time needed to take action when a blood transfusion is necessary [12]. The statistical analyzes performed did not show any influence of the ABO blood group on the occurrence of pre-eclampsia; the differences in the number of examined women within individual groups of pregnant women were not statistically significant. However, significance was demonstrated in the case of the Rh factor. It has been shown that the Rh- blood group is more common in women with preeclampsia than in women in the control group. Scientific research does not clearly determine the influence of the Rh factor on the occurrence of pre-eclampsia, which should prompt further observations in this direction. In their studies, Burgess et al. noted an increased incidence of late-onset preeclampsia, and blood group B observed an increased incidence of early-onset preeclampsia [13].
Anemia is a pathological condition in which the hemoglobin level is too low compared to the reference values. When assessing pregnant women in this respect, one should take into account the physiological differences related to the adaptive changes occurring during pregnancy in blood morphology parameters compared to healthy women of reproductive age. This knowledge will allow for the correct classification of patients into the group of women with anemia, normal hemoglobin content and polycythemia. Due to the increased demand of pregnant women for particular nutrients, deficiency anemia is most often observed during pregnancy. Hemolytic anemia associated with preeclampsia also occurs during pregnancy. A strong relationship between the occurrence of EPHgestosis and anemia was observed in the study population. These results were statistically significant. Similar relationships were revealed in a study conducted by Mahmood et al. [14]. It is therefore important to pay attention to hemoglobin concentration in pregnant women.
An important test during pregnancy is the assessment of urine sediment. Proteinuria is one of the diagnostic criteria for preeclampsia. However, the examination of urine sediment also has other elements, the importance of which in predicting preeclampsia was also examined. The obtained statistical data prove that the variability of the assessed elements, i.e. erythrocytes, leukocytes and bacteria in urine, is not related to the occurrence of preeclampsia. Laboratory test results in this regard are similar in both groups. Similar conclusions follow the research conducted by Girsberger et al. [15].
Conclusions
In the studied population, the Rh factor had statistical significance. It is important to expand the research group to clearly determine whether blood type and the Rh factor influence the occurrence of preeclmapsia. This will make it possible to classify patients into increased risk groups and introduce activities aimed at early detection of indicators that may suggest the occurrence of EPH-gestosis.
Hemoglobin concentration is an important predictor of preeclampsia in pregnant women. A noticeably larger number of anemic patients experience preeclampsia, which undoubtedly proves their coexistence in the pathomechanism of EPH-gestosis.
The research should be extended to a larger group of patients and the number of parameters assessed should be increased in order to more precisely assess the tested parameters and verify the results obtained in the preliminary reports.

 POLSKI
POLSKI