Introduction
Longevity is rightly considered one of the greatest achievements of modern society and a key public health challenge is to ensure citizens remain healthy and disability-free as long as possible. The increase in life expectancy and global population ageing have resulted in substantial challenges to societies and health systems [1,2]. On the topic of longevity and ageing, it is important to make a distinction between old, nonagenarians, and centenarians. Under particularly favourable conditions, including an average temperature of around 20⁰C, many hours of sunshine, an ecological environment, strong family and local inhabitants’ ties, and use of traditional/local food, some groups of humans demonstrate the ability to exceed 100 years of age, in fair health [2]. In Poland, with a temperate climate with cold winters, inhabited by approx. 37.7 million people there are no so-called “(Longevity) Blue Zones” and the centenarian ratio is <0.7 with more than three times more women than men among centenarians [3,4]. In 2020 actual life expectancy at age 65 was 14.5 years for men and 19.2 for women [5]. Data from the Swedish Twin Registry showed that the effect of genetic inheritance on life expectancy accounts for 20-30% and almost all of the remaining variance is due to non-shared, individual-specific factors [6]. It is strongly suggested that longevity is an excellent result of an optimal performance of the immune system related to an overexpression of anti-inflammatory variants in immune/inflammatory genes, which does not however exclude the participation of other systems or organs [1]. In the pandemic, the threat of a turbulent course of COVID-19 and the risk of death was lower in women after adjustment for age and comorbidities [7]. Women usually have stronger innate and adaptive immune responses than men, which makes a faster clearance of pathogens and greater vaccine efficacy [1].
Older residents in nursing homes or long-term facilities are at greatest risk of morbidity and mortality from SARS-CoV-2 infection, starkly highlighted in the first wave of the COVID-19 pandemic where older nursing homes represented one-third to more than half of all deaths [8]. Breakthrough infections and outbreaks continued to occur in 2021 and the beginning of 2022 in nursing home residents previously vaccinated, increasing in number and scale before the rollout of booster vaccine doses [9]. People in these centres were prioritised for SARS-CoV-2 vaccination and most residents in nursing homes in Poland received full vaccination with recommended booster doses. Testing humoral immunity is the common means of determining past infection and/or vaccination, but it has some important caveats [10,11]. Cellular immunity, based on T-lymphocyte responses, is a key element to sustain prolonged immunological response [12,13]. A mounting body of evidence points to the importance of evaluating T-cell responses, including the detection of excreted interferon-γ (IFN-γ) as an established blood-based marker of T-cell activation, alongside humoral responses when assessing immunological status for individuals at greater risk of severe COVID-19 [14]. Unfortunately, cellular assays are both expensive and cumbersome compared to serological ones.
In individuals older than 65 years SARS-CoV-2 infection may contribute to the loss of coordinated response between the cellular and the humoral responses [12]. In this study, we aimed to measure the immunological cellular response to an mRNA-based SARS-CoV-2 vaccine by applying a commercial whole-blood-based IFN-γ release assay (IGRA) and correlating it to the humoral response in female nonagenarians and other elder residents of nursing homes located in a rural area of South-East Poland, where, without exception, the residents were exposed to multiple outbreaks of COVID-19.
Materials and methods
Study population and ethical issues
For the present study, three doses vaccinated with the BNT162b2 vaccine (Comirnaty®, Pfizer-BioNTech) residents from the three nursing homes for older people in Biała Podlaska county, South-East Poland, were invited. Participation in the study was voluntary and included self-written informed consent of mentally competent individuals. Demographic information was collected at enrolment. The exclusion criteria were any kind of immunomodulatory therapy or any medical condition that may significantly lower the participant’s immunity. The study protocol was approved by the local Ethical Committee. The authors assert that all procedures contributing to this study are compatible with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
SArS-cov-2 antibodies determination
Blood samples were immediately transferred to the laboratory, where IgG antibody titters to the SARSCoV-2 S1-protein in serum specimens were determined using the Anti-SARS-CoV-2 QuantiVac ELISA IgG assay (Euroimmune, Lübeck, Germany) according to the manufacturer’s instructions. The ELISA is based on 96-well microplates coated with the SARS-CoV-2 S1 domain (including receptor-binding domain, RBD) expressed recombinantly in the human cell line HEK293. Quantification of S1-specific IgG was performed using a 6-point calibration curve covering a range from 1 to 120 relative units (RU)/ mL. Samples yielding results in the analytical range were re-evaluated at a higher dilution. Positive and negative controls were included in each test run. By multiplication with factor 3.2 results in RU were converted into standardised binding antibody units (AU/mL). Seropositivity was defined as ≥ 35.2 AU/mL.
Ex vivo ifn-γ-release assay
For any single participant, whole blood was collected in lithium heparin tubes, and within 2 h from venepuncture five hundred μL was placed into three separate tubes (SARS-CoV-2 IGRA Euroimmun, Lübeck, Germany): (1) IGRA Blank, a tube containing no activating components for immune system for the determination of individual background IFN-γ levels, (2) IGRA Stim, a stimulation tube coated with mitogen for non-specific T-cell stimulation as an unspecific control for the viability and stimulation ability of T-cells, and (3) IGRA Tube, a stimulation tube coated with components of the S1 domain of SARS-CoV-2 Spike protein able to stimulate specific CD4+ and CD8+ T lymphocytes. Plasma was harvested after 24 h of stimulation at 37⁰C from each tube and evaluated for IFN-γ concentrations on an IFN-γ ELISA plate, according to the manufacturer’s instructions. Quantification of IFN-γ was performed using a six-point calibration curve covering a range from 0.1 to 428 mIU/ mL. Samples yielding results in the analytical range were re-evaluated at a higher dilution. Two controls were included in each test run. The results above the concentration of 17120 mIU/mL were considered equal to 17120 mIU/mL. The patient result was considered valid when IFN-γ values were < 400 mIU/mL in IGRA Blank and > 400 mIU/mL in IGRA Stim. The actual IFN-γ output for every participant was defined as the IGRA Tube value minus the value from the respective IGRA Blank. Results <100 and >200 mIU/mL were considered negative and positive, respectively. The manufacturer defined a grey zone between 100 and 200 mIU/mL, and the results within this zone were interpreted as positive in the context of a high positive predictive value in SARS-CoV-2 exposed and vaccinated participants.
Statistical analysis
Statistical analysis was performed by using the Polish version of the TIBCO Software Inc., USA (2017). The compliance of the data with the normal distribution was assessed using the Shapiro-Wilk test. Categorical data were compared using the chi-square or Fisher exact test. The comparison of proportions between groups was performed using the Mann–Whitney U test. Correlation between continuous variables was performed by using Spearman’s rho test. The statistical significance was accepted as p < 0.05.
Results
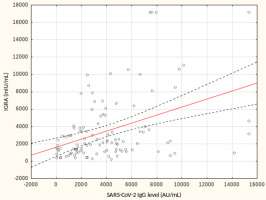
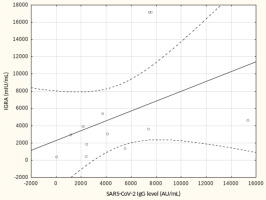
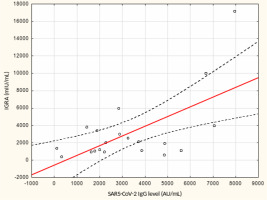
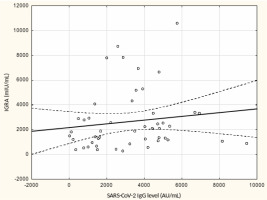
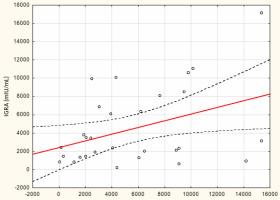
From May to September 2022 a total of 109 female subjects living as residents in three nursing homes for elder people were tested. Figure 1 shows the primary characteristics of residents included in the study. The median (interquartile range, IQR) age was 76.0 (71.0 – 83.0) years. Forty-four percent of the study participants were aged 70-79 years. There was not a single centenarian in the recruited group, but 12 nonagenarians (11%). Except for one 82-year-old female with borderline IGRA result of 193.8 mIU/mL coexisting with SARS-CoV-2 IgG of 1376.8 AU/mL, no cases showed a lack of borderline values of humoral or cellular response. Median levels (IQR) of SARS-CoV-2 IgG and IGRA were 3496.3 (1681.9-5363.8) AU/ mL and 2100.0 (1144.4-3925) mIU/mL, respectively. There were no statistically significant differences in the concentration of anti-SARS-CoV-2 antibodies and IGRA between nonagenarians and participants from younger age groups, Table 1. We found a moderate statistically positive correlation between concentrations of SARS-CoV-2 IgG and INF-γ in IGRA, Figure 2. The level of correlation between SARS-CoV-2 IgG antibodies and IGRA was similar in nonagenarians as in the entire study group, r = 0.406 vs. r = 0.408 (Figure 2 and Figure 3). Correlations between anti-SARS-CoV-2 antibodies and IGRA in other selected age groups are presented in Figures 4, 5 and 6. The lowest level of correlation was observed in the eighth decade of life and the highest in participants aged 65-69 years (Figures 6 and 4).
Table 1
Characteristics of participants by age and immune response
Discussion
Now that the SARS-CoV-2 virus has been circulating worldwide for nearly four years, the heightened and robust protection is afforded by a combination of naturally acquired infection and vaccination (hybrid immunity), which has become increasingly apparent especially in residents of nursing homes, as supported by immunological and epidemiological evidence [15]. Generally assessing both humoral and cellular responses together is of interest to patient management as it may help identify the best candidates for either revaccination or other prophylactic therapy [11,16]. However, in the three-times vaccinated female nursing home residents we studied, including a group of 12 nonagenarians, we did not find a lack of cellular response to the S1 domain of SARS-CoV-2 Spike protein in the IGRA test. These data emphasise that special additional immunisations for at least female individuals aged 90+ in nursing homes with normal anti-SARS-CoV-2 antibody levels, which are easy to test, might not become necessary (in that period). Of course, nursing home residents are a very specific population due to their extreme vulnerability to COVID-19 and this makes our observation of nonagenarians likely to be of value when designing prevention. Hardy et al. [17] showed that the overall death rate from COVID-19 among Belgian nursing home residents before vaccination started was 130 times higher than outside. Unfortunately, we do not have data on the number of deaths during the pandemic in nursing homes, where we had recruited study participants, which could give a picture of the degree of pandemic selection.
The exact levels of antibodies and cellular responses to protect against COVID-19 in older people are still unknown, however, we know that cellular immunity does not wane and remains up to 20 months after the last contact with the viral antigen in vaccinated individuals and patients with previous infection, as well as, INF-γ presumably plays an important role not only in T-cell activation and early protective response to COVID-19 disease, immune protection from severe COVID-19, but also in preventing SARS-CoV-2 infection and SARS-CoV-2 reinfection after vaccination [13,18]. The essentiality of INF-γ contribution for vaccine-induced protection upon SARS-CoV-2 infection was additionally clearly demonstrated in animal models, including non-human primates [19,20].
Recently, Pinti et al. [21] analysed cytokine networks in 22 Italian healthy centenarians and reported no relevant difference in non-stimulating concentration of INF-γ, but increased pro-inflammatory INF-α, in comparison to healthy middle-aged controls. Similarly, to our results mostly weak correlation between anti-RBD antibody titters and INF-γ in the IGRA was reported in different groups of healthy individuals vaccinated with the BNT162b2 vaccine (e.g., Polish employees in the social care delivery institution) and patients with diseases of various etiologies [11,22-24]. So far, very few studies investigated humoral and cellular immunity, using IGRA, in older nursing home residents and there are no studies devoted to individuals aged 90+. Van Praet et al. [25] found a correlation between anti-SARS-CoV antibodies and IGRA in 100 Belgian residents of long-term facilities, who were vaccinated with the BNT162b2 vaccine. Meijide-Miguez et al. [15] and San Roman et al. [26] reported the IGRA test performance without a full description of age and results in 20 and 28 old residents of nursing homes vaccinated with the BNT162b2 vaccine, respectively.
The current study has notable strengths. The population under investigation was ethnically homogenous and from the same rural area. Participants came from facilities run by local governmental authorities with a relatively low level of funding and with comparable standards of care and protection against SARS-CoV-2 transmission. In these nursing homes, 24/7 medical supervision is provided by nurses. It should be stressed that recruited nonagenarians came from rather large communities of co-inhabitants and not from places where they could be provided with particularly specialised care. There is some evidence linking nursing homes’ quality, architectural design access to outdoor space, ventilation, diet offer, staffing, and for-profit status with the likelihood of outbreaks and mortality in the ongoing pandemic [27]. The obvious limitation of our study is a relatively small size sample and therefore conclusions should be taken with caution. The correlation between humoral and cellular immunity, although statistically significant in some samples, as well as in our whole group of participants, is still weak, which may be in part due to the heterogeneity in the population, particularly concerning comorbidities. We assessed the humoral response with only one type of antibodies against SARS-CoV-2 and the cellular response only with IGRA, while INF-γ is only one of the components, albeit crucial, of the cellular response [12,16]. However, several studies documented a significant correlation between anti-spike (S1 domain) immunoglobulins G (IgG) (measured by routine commercial tests) and neutralising titters, suggesting that IgG antibodies might serve as a correlate of SARS-CoV-2 neutralisation [10].
Long-term care facilities for elder people face numerous challenges. Several studies show that to meet the current and future needs in care for elders, the contribution of qualified nurses interested in improving work methods and conducting scientific research is essential [28]. The presented work was carried out by a nursing research group.
In conclusion, we found similar levels of anti-SARS-CoV-2 IgG antibodies and post-stimulatory INF-γ in female nonagenarians and elder residents of nursing homes vaccinated with the BNT162b2 vaccine. The presented results do not constitute an argument for the routine performance of the IGRA tests in nonagenarians, especially in financial resource-limited settings.

 POLSKI
POLSKI