Introduction
It has been observed that a group of conditions exert a detrimental impact on the well-being of community members. This cluster of conditions coincides with a decrease in the birth rate among females and fertility in males. These conditions, among others, include obesity, hypertension, hyperglycemia, and hyperlipidemia, which can collectively be classified as metabolic syndrome [1-2]. Infertility, which is defined as the absence of fertilization after a year of regular intercourse, is a pervasive global health challenge affecting both genders. While some causes of infertility are gender-specific, others are not. Azospermia refers to the absence of sperms in the semen, while Oligospermia is a condition characterized by a decreased number of sperms in the semen, with less than 15 million sperm/ml. Severe Oligospermia, on the other hand, is defined as a condition where the number of sperms in the semen is less than 5 million sperm/ml. Male fertility is affected by various factors, including those that are related to the structure of the reproductive system, general disorders (physiological, immunological, and hormonal), genetic factors, as well as physical factors such as testicular trauma. The occurrence of infertility is also attributed to varicocele and testicular cancer [3-7]. Immuno-infertility is a phenomenon whereby antibodies to sperms (Anti-sperm antibodies) are formed in semen. The generation of these antibodies culminates in a reduced ability of sperm to penetrate cervical mucus and attach to oocytes, thereby hindering fertilization and fetal development. Ultimately, male fertility and progeny levels are diminished [8-9]. Human semen is composed of two parts; the first is cellular, comprising mature and immature sperm cells, epithelial cells of the urogenital tract, and leukocytes, The second part is non-cellular and is constituted by secretions from male reproductive glands (Testes, Epididymides, Prostate, Seminal vesicles, Cowper’s and Littre glands) encompassing organic substances (proteins, sugars, lipids) and inorganic ions such as zinc [10]. The absorption of calcium by the intestines is a fundamental and essential process for various metabolic functions in the human body. Vitamin D plays a crucial role in promoting bone health by enhancing the absorption of calcium by the intestines. The body acquires vitamin D from two primary sources, namely exposure to sunlight and the ingestion of vitamin D from food and supplements. The inadequate concentration of vitamin D is a leading cause of autoimmune diseases, such as rheumatoid arthritis [11-13]. Numerous studies have reported that vitamin D deficiency in men results in reduced sperm motility compared to those with normal vitamin D levels [14]. Additionally, a hypothesis suggests that vitamin D is crucial in transporting calcium into the epididymis [15]. Recent research has confirmed that a positive relationship exists between vitamin D and male reproductive hormones, thereby enhancing reproductive ability [16-17]. These studies support the notion that Vitamin D levels play an essential role in semen quality[18]. The Anti-Müllerian hormone (AMH) is a glycoprotein secreted by Sertoli cells and belongs to the transforming growth factor β (TGF-β) family. AMH has an important role in determining the sex of the fetus. The concentration of AMH decrease at puberty [19-20]. There is a study that demonstrates a positive correlation between the level of serum AMH and inhibin B, FSH, and the size of the testis, but not with the concentration of sperms [21]. A recent investigation has divulged that a reduction in AMH present in the serum is a distinctive indication of Sertoli cell dysfunction and inadequate sperm production among infertile men. Additionally, the study observed that no correlation exists between AMH and vitamin D levels, and that the introduction of vitamin D supplementation does not alter the concentration of AMH [22]. Lipids play a prominent role in the activation of the cellular stress pathway. The dissolution of lipids in the cell membrane results in the peroxidation of lipids, which are considerably destructive to cells, such as aldehydes, leading to the development of oxidative stress. It has been scientifically established that oxidative stress is a contributing factor to the escalation of male infertility issues [23-24]. A previously conducted study has demonstrated the influential role of lipids in male and female fertility. The enhancement of oocyte and fetus in females, as well as the significant aspects of sperm formation in males (Spermatogenesis), are correlated with the increase of high-density lipoprotein (HDL). The negative effects on the functionalities of the reproductive system, specifically the mechanism of ejaculation and the quality of semen, are caused by the damage to the nDNA and mtDNA of the sperms, which is associated with diabetes. The treatment of diabetes mellitus Type-1 solely relies on insulin, and the inadequacy of insulin may lead to hypogonadism, ultimately resulting in a decrease of fertility [25-27]. In the current study, the variation in some immuno-physiological markers for patients with Azospermia, Oligospermia is compared to Normospermia.
Materials and methods
2.1. Ethical research
The investigation was conducted at the Bab Al-Moadham of the Medical City Hospitals in Baghdad Governorate. The medical laboratory science departments’ scientific committee and the director of the research Centre at the University of Raparin thoroughly scrutinized and approved the protocol and procedures of the present study.
2.2. Experimental design
The present investigation encompassed two parts. Firstly, the immune facet was explored, whereby various immunological markers were estimated. Secondly, certain physiological parameters were investigated. The study consisted of 140 volunteers, who were categorized into three groups: Group A (Normospermia; n = 50), Group B (Oligospermia; n = 45), and Group C (Azospermia; n = 45).
2.3. Methods
The samples were collected from participants at laboratories of Medical City Hospitals /Baghdad-Bab Al-Moadham. The centrifugation at 1000 g for 35 minutes at room temperature was used in the separation of the sera from the blood. The sera were prepared and held at a temperature of (-20°C). The kits used in present study are Vitamin D, AMH, Testosterone C-RP, RF, Cholesterol, Triglyceride, LDL, VLDL, Insulin, FBS, and HbA1c. A cobas e 411 analyzer was used to detect the concentration of vitamin d and insulin, while AMH and testosterone were measured by using The Sandwich Enzyme-Linked Immune-Sorbent assay (ELISA). A spectrophotometer (Enzymatic colorimetric method) was used to estimate FBS and Lipid profiles. CRP, RF, and, HbA1c were tested by using Abbott Architect c4000 analyzer.
2.4. Statistical analysis
The statistical analysis was executed through the utilization of the software package, Statistical Package for Social Science (SPSS) V20. A one-way analysis of variance, also known as ANOVA, was conducted. It was determined that a P value of 0.05 was statistically significant for all the findings.
Results
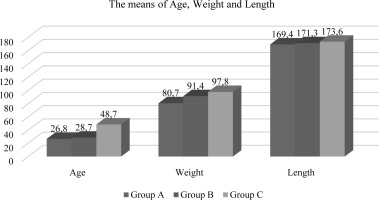
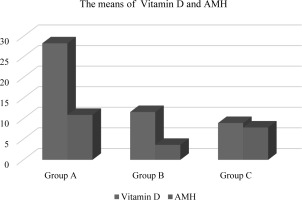
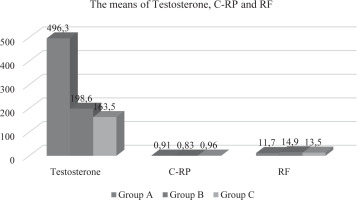
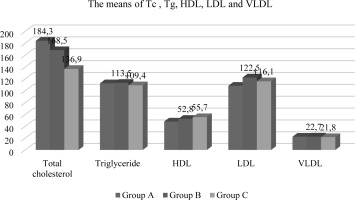
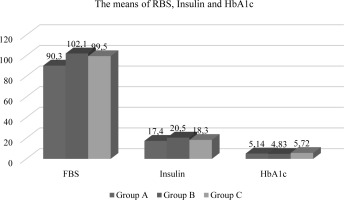
In the present investigation, a statistically significant association (p<0.05) was observed among the groups included with respect to age and weight, while no noteworthy differences were observed for length. The mean age values for Oligospermia and Azospermia were determined to be 28.8±6.82 (years) and 48.7 ± 9.33 (years) respectively, as compared to the mean value of Normospermia which was found to be 26.8 ± 5.11 years (refer to Table-1 and Figure-1). Similarly, for weight, the average value of Normospermia was found to be 80.7 ± 14.3 (kg), whereas the mean values of Oligospermia & Azospermia were calculated to be 91.7 ± 11.3 and 97.8 ± 13.6 (kg) respectively (refer to Table-1 and Figure-1). In the current study, the mean length of Normospermia was established to be 169.4± 12.4 (cm), while the corresponding values for Oligospermia and Azospermia were found to be171.3±14.5; 173.6±8.48 (cm) respectively (refer to Table-1 and Figure-1). Moreover, the findings of our investigation demonstrated that the averages of Vitamin D for cohorts A, B, and C amounted to 28.3 ± 6.13 (ng/mL); 11.6 ± 4.72 (ng/mL); 8.93 ± 1.83 (ng/mL), respectively (refer to Table-2 and Figure-2). In comparison to the value observed in Normospermia, the result of Oligospermia for AMH was 3.63 ± 1.38 (ng/mL), while Azospermia was 7.84 ± 1.81 (ng/mL) (refer to Table-2 and Figure-2). As a consequence, our current study divulged that the averages of testosterone for groups A, B, and C were 496.3±31.1 (mg/dL); 198.6 ± 28.9(mg/dL); 163.5± 17.4 (mg/dL), respectively (refer to Table-3 and Figure-3). The mean concentration of C-RP was found to be 0.83±0.19 (mg/dl) for Oligospermia, and 0.96 ± 0.08 (mg/dL) for Azospermia (Table-3 and Figure-3), in contrast to Normospermia where it was observed to be 0.91±0.03 (mg/dl) (Table-3 and Figure-3). The current investigation revealed that the mean concentration of RF for Normospermia was 11.7± 1.82 (IU/mL) (Table-3 and Figure-3), while for Oligospermia and Azospermia, the mean values were 14.9 ± 1.58 (IU/mL) and 13.5±1.47 (IU/mL) respectively (Table-3 and Figure-3). Table 4 and Figure 4 illustrate the average values of the lipid profile. For Group A, the mean concentrations of Cholesterol, Triglyceride, HDL, LDL, and VLDL were found to be 184.3±22.6, 112.7±19.1, 48.3 ±11.5, 108.5 ±16.4, and 22.5 ±9.31(mg/dL), respectively. Similarly, Group B had mean levels of Cholesterol, Triglyceride, HDL, LDL, and VLDL at 168.5±26.8, 113.5±24.1, 52.8 ±13.8, 122.5 ±19.3, and 22.7 ±6.82(mg/dL), respectively. Group C, on the other hand, exhibited an average of Cholesterol, Triglyceride, HDL, LDL, and VLDL at 136.9± 21.6, 109.4± 17.9, 55.7 ±14.6, 116.1 ±13.9, and 21.8 ±8.38 (mg/dl). Respectively, The study has demonstrated that the mean values of RBS for Normospermia, Oligospermia, and Azospermia were 90.3 ± 11.8(mg/dL), 102 ± 13.2(mg/dL), 99.5 ± 8.63(mg/dL), respectively. In addition, the mean values of HbA1c for the three groups (A, B, and C) were 5.14± 1.34%, 4.83± 0.55%, and 5.72± 0.38 %, respectively. Finally, it was observed that the mean value of Oligospermia for insulin was 20.5 ± 5.37(IU/mL) and that of Azospermia was 18.3 ± 3.86 (IU/mL) as compared to the level of Normospermia, which was 17.4 ± 3.74(IU/mL). Table and Figure (5) present the means of RBS, HbA1c, and insulin for the three groups. A correlation was observed between anti-Müllerian hormone (AMH) and various factors including age, vitamin D levels, total cholesterol, high-density lipoprotein (HDL) cholesterol, random blood sugar (RBS), insulin, and hemoglobin A1c (HbA1c) (Table-6).
Table 1
The mean values of age, weight and length
Table 2
The mean values of vitamin D and AMH
| VARIABLES | GROUP A N=50 | GROUP B N=45 | GROUP C N=45 | TOTAL N=140 | P-VALUE |
|---|---|---|---|---|---|
| Vitamin D/(ng/mL) | 28.3 ± 6.13 | 11.6 ± 4.72 | 8.93 ± 1.83 | 16.27 ± 4.22 | 0.0113 |
| AMH (ng/mL) | 10.9 ± 2.92 | 3.63 ± 1.38 | 7.84 ± 1.81 | 7.45 ± 2.03 | 0.0241 |
Table 3
The means values of Testosterone, C-RP and EF
Table 4
The mean values of lipid profile (mg/dL)
Table 5
The mean values of RBS, insulin and HbA1c
Table 6
The correlation between AMH and other parameters
Discussion
It has come to attention that a combination of factors has a harmful influence on the health of community members. This grouping of circumstances, among others, include obesity, hypertension, hyperglycemia, and hyperlipidemia, which can collectively be categorized as metabolic syndrome [1-2]. While certain causes of infertility are specific to one gender, others are not. The term azospermia refers to the absence of sperm in the semen, while oligospermia is a condition characterized by a reduced number of sperm in the semen, with less than 15 million sperm per milliliter. On the other hand, severe oligospermia is defined as a condition where the number of sperm in the semen is less than 5 million sperm per milliliter. Male fertility is influenced by various factors, including those related to the structure of the reproductive system, general disorders (physiological, immunological, and hormonal), genetic factors, as well as physical factors such as testicular trauma. The occurrence of infertility is also attributed to varicocele and testicular cancer [3-7]. Varicocele (VC) is observed in approximately 35-40% of males experiencing infertility. Nevertheless, theexisting surgical interventions and antioxidant therapies do not yield comprehensive efficacy. Aside from the presence of oxidative stress, it is probable that other variables, including the perturbation of the immune microenvironment within the testicles, play a role in the irrevocable impairment of testicular function. Indications propose a correlation between VC and the occurrence of anti-sperm antibodies [28]. Abnormal semen parameters are a significant factor in male infertility, with genetic disorders playing a leading role among the many etiological factors. Spermatogenesis regulation is dependent on a range of genes located on both the Y chromosome and autosomes, which exert their influence at various stages of germ cell development [29]. According to the World Health Organization, various environmental factors have been noted to impact reproductive health through their interference with the organism’s physiological functions [30]. The association between obesity and testosterone deficiency has been substantiated by a multitude of studies [31-33]. It has been observed that testosterone levels in overweight males are frequently comparable to those observed in hypogonadal men. The activity of the aromatase enzyme in adipocytes is amplified in obese men, causing the peripheral conversion of testosterone to estradiol [34-36]. Based on the evidence that VD is metabolized in the human testis and ejaculatory tract, there has been an increasing recognition of the significant role played by local activation of VD in spermatogenesis and sperm maturation [15]. The lipid composition in the sperm membrane and seminal plasma has been shown to be significantly associated in various investigations. The seminal plasma of patients with oligospermia and azoospermia has been found to contain higher levels of phospholipids. Moreover, an analysis of the lipid composition of the human testis in individuals with bilateral varicocele as a cause of infertility has demonstrated the abundance of lipids in the testis tissue [25]. The management of Type-1 diabetes mellitus is exclusively dependent on insulin, and the insufficiency of insulin can potentially give rise to hypogonadism, ultimately culminating in a reduction in fertility [25-27]. Diabetes Mellitus is linked with an increase in damage to both sperm nDNA and mtDNA, leading to adverse effects on the reproductive capabilities of affected men. Moreover, it also negatively impacts the quality of sperm and the process of ejaculation [26]. The current study has revealed that individuals with Azospermia exhibit higher age and body weight, as well as greater mean height in comparison to the control group (Normospermia). In terms of vitamin D and AMH levels, the concentration for Group C (Azospermia) and Group B (Oligospermia) was lowers as compared to Group A (Normospermia). Furthermore, the study has demonstrated that the differences among the three groups are highly significant with respect to the concentration of testosterone. A noticeable decline in the concentration of the hormone was observed in patients with Azospermia and Oligospermia as compared to the control group. Additionally, the level of CRP did not differ significantly between the patients and the healthy individuals (Control group). Regarding RF, while there was a rise in the concentration of groups B and C as opposed to group A, the increase was not statistically significant. Lipids exert a significant influence in the initiation of the cellular stress pathway. The solvation of lipids within the cellular membrane yields the peroxidation of lipids, which possess a considerable capacity for cellular detriment, exemplified by the presence of aldehydes, thereby instigating the emergence of oxidative stress. It has been scientifically validated that oxidative stress serves as a contributory element in the exacerbation of male fertility complications [23-24]. In reference to the lipid profile elements, including Cholesterol, Triglyceride, HDL, LDL, and VLDL, the outcomes demonstrated normal values for all samples. Additionally, it was discovered that the decline in concentration of Cholesterol and Triglyceride was gradual for groups A and B, respectively. With regards to HDL, an elevation was observed in groups B and C in relation to group A. However, the changes in LDL and VLDL were negligible for all groups involved in the current investigation. The results pertaining to RBS, insulin, and HbA1c were found to be within the normal range in the study. Notably, no discernible trend in terms of upward or downward progression was observed between the groups. However, the investigation did demonstrate that the observed changes were uncomplicated and did not reach the threshold of statistical significance. Lastly, a correlation was identified between anti-Müllerian hormone (AMH) and diverse variables encompassing age, levels of vitamin D, total cholesterol, high-density lipoprotein (HDL) cholesterol, random blood sugar (RBS), insulin, and hemoglobin A1c (HbA1c). These correlations underscore the significance of these examinations in the early detection or containment of the disease.
Conclusion
Our research has demonstrated that the detection of disorders in the male reproductive system necessitates the assimilation of both immunological and physiological indices. A marked discrepancy in the levels of the aforementioned indices was observed afflicted with Aligospermia and Azoospermia relative to those with Normospermia.

 ENGLISH
ENGLISH