Introduction
Due to the increasing incidence of endometrial cancer worldwide and the results remaining at a comparable level despite the progress that has been made in medicine in recent years, this cancer is the subject of intensive research. The incidence of this type of cancer is highest in highly developed countries, where it is the most common cancer of female organs [1]. Since the beginning of the 21st century, the epidemiology of EC in Poland has begun to resemble that observed in the world. In the light of research on this type of cancer, the key role of diet and nutrients in the process of shaping this disease is highlighted. Reports from recent years indicate that natural nutritional products have preventive or therapeutic potential in The fight against these diseases. The influence of environmental factors, lifestyle, and exposure to pollution does not go unnoticed in the case of this disease. Improper eating habits among women of reproductive age contribute to a growing tendency to morbid obesity, metabolic diseases, diabetes and polycystic ovary syndrome (PCOS), which in the long run may escalate the risk of endometrial cancer. Obesity, diabetes and overweight are unfavorable factors for invasion and metastasis [2,4,5].
In the context of this disease, lymphovascular space invasion (LVSI) is an important clinical prognostic factor. The role of LVSI in relation to The risk of disease recurrence and the selection of appropriate treatment remains controversial and unclear. Different researchers express different opinions on this topic and there are significant discrepancies between Them. The study aims to summarize the current knowledge and scientific research on LVSI, or its lack, as the strongest independent prognostic factor in the case of regional recurrence in the minor pelvis, distant metastases and overall survival [5].
Material and methods
The study included 306 Caucasian women diagnosed with confirmed endometrial cancer at the WSZZ in Kielce between 2005 and 2017. The patients were operated on in two facilities: WSZZ in Kielce, 103 patients (Group 1) and 206 patients at ŚCO (Group 0). The patients underwent total hysterectomy with pelvic lymphadenectomy, computed tomography of the abdomen and chest, typical blood tests and endoscopic examination. Each case was rediagnosed according to the eighth edition of the TNM classification [6,7]. All subjects underwent typical surgical treatment without prior radio-chemotherapy. This resulted in a reliable comparative analysis of tumor characteristics in terms of treatment and an unchanged molecular profile. Data on demographic variables and pathological findings were collected. The data included patient age, comorbidities, body mass index (BMI), comorbidities such as diabetes, history of hypertension, thyroid disease, clinical stage (FIGO), pathology stage, lymphovascular space invasion (LVSI) and estrogen receptor expression levels ( ER), progesterone receptor (PR) and Ki67 in EC tissue (in patients operated on at WSZZ in Kielce) [6]. The stage of cancer advancement was classified according to the 2009 classification of the International Federation of Gynecology and Obstetrics (FIGO) [16,17]. Tumor stage and histological classification were assigned based on the 2003 WHO classification of tumors [5]. Types of endometrial cancer were identified based on the Bokhmann Classification [6] A basic panel of biochemical tests was performed.
Statistical analysis
Quantitative data were presented as mean and standard deviation or median and range. Categorical data were expressed as numerical and percentage distributions. The chi-square test or Fisher’s exact test was used to compare proportions, and a multivariable logistic regression model was used to assess the association between target genes. Follow-up time was calculated as the number of years from surgery to disease recurrence, death from causes other than cancer, or last contact with the patient. To identify an independent prognostic factor for disease-free survival, a multivariable Cox proportional hazards model with backward selection (with a cutoff of 0.15) was performed on variables that were statistically significant in the univariate analysis.
All statistical tests were two-sided, and values <0.05 were considered significant. Calculations were performed using STATISTICA (data analysis software system), StatSoft, Inc. (2014), version 12, Tulsa, OK, USA, available online: http://www.statsoft.com/, accessed May 2, 2022.
Results
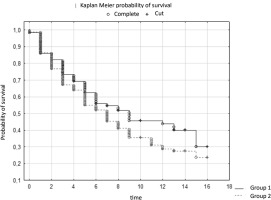
The study involved 306 patients aged 41 to 93. Survival analysis was performed taking into account the variable surgery. The results of the log-rank test indicate statistically significant differences in survival between groups of patients. The test statistic values are WW = 38.419, Rank Sum = 152.73, Rank Variance = 33.939, Test Statistic = 6.594758, and p-value = 0.00000.
These values are significant because the p-value is 0.00000, which is well below the established significance level (usually 0.05). This means that there are significant differences in survival between the groups of patients who underwent surgery and those who did not. The positive value of the log-rank test statistic (6.594758) suggests that patients who underwent surgery tended to have better survival than those who did not undergo surgery. These values indicate significant differences between groups in the context of survival time, which may be important from the point of view of assessing the effectiveness of surgical intervention in the context of the examined issue
Table 1
Cox’s Hazard
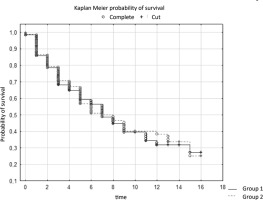
In the study, the survival time of patients in two different centers depended on the clinical stage of the cancer. The survival time of patients from groups G1-G3 was analyzed. The results of the log-rank test indicate significant differences between the groups. The test statistic values are WW = -10.01, Rank Sum = 152.73, Rank Variance = 37.319, Test Statistic = -1.63889, and p-value = 0.10124.
These values are significant because they are below the established significance level (usually 0.05). This means that there are significant differences in survival between G1 patients at both study sites. A negative log-rank test statistic suggests that patients in one center tended to have a longer survival time than those in the other center. The survival time of patients in both centers depended on the clinical stage of the cancer (Table 2).
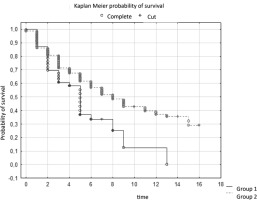
In the context of survival of patients with stage G2, analysis was performed using the log-rank test. The results of this test showed no statistically significant differences between the patient groups. The test statistics values are WW = 0.96494, Rank Sum = 152.73, Rank Variance = 37.151, Test Statistics = 0.1583127, and p-value = 0.87421.
A p value above the established level of significance (usually 0.05), i.e. p = 0.87421, indicates no statistically significant differences between groups in the context of survival of G2 patients. The log-rank test statistic, which is a measure of the difference between survival curves, is close to zero (0.1583127), which confirms the lack of significant differences between the groups. (Table 3)
In the analysis of survival time of patients from group G3 stage of endometrial cancer, the log-rank test was performed. The results of this test show statistically significant differences in survival time between both centers. The test statistics values are WW = 10.854, Rank Sum = 152.73, Rank Variance = 19.625, Test Statistics = 2.450104, and p-value = 0.01428.
A p-value of 0.01428 is lower than the established significance level (often 0.05), indicating statistical significance. This means that there are significant differences in survival between two centers with the same G3 cancer stage. The positive value of the log-rank test statistic (2.450104) suggests that there is a difference between the groups in terms of survival time. To sum up, the results of the analysis indicate that the stage of endometrial cancer in the G3 group affects the survival time of patients, which may be important from the point of view of prognostics and the approach to treatment depending on the stage of the disease.
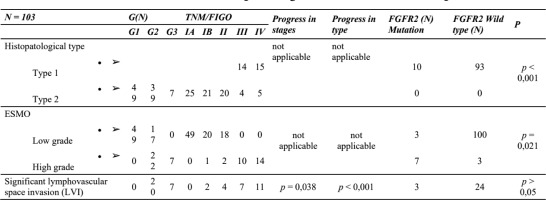
Significant lymphovascular space invasion (LVSI) was defined as ≥4 LVSI-positive vessels on at least one H&E slide. Among the patients in group 1, additional tests were performed to confirm the presence of LVSI
Tumor grade progression was closely correlated with LVSI (R = 0.2113, p = 0.0338), vimentin expression (R = 0.5344, p = 0.000), tumor budding (r = 0.4867, p = 0.000), B-catenin (R = 0.410, p = 0.044) and lack of E-cadherin (R = 0.2950, p = 0.0028). Similar observations were noted with respect to TNM/FIGO – LVSI stage progression (R = 0.6949, p = 0.000), vimentin expression (R = 0.2573, p = 0.009), tumor budding (R = 0.3098, p = 0.000), B-catenin (R = 0.437, p = 0.032) and no E-cadherin (R = 0.3291, p = 0.000).
Table 6
Comparison of univariate and multivariate Cox models
| Univariate Cox model | Multivariate Cox model | |||||||
|---|---|---|---|---|---|---|---|---|
| Plac Chi | HR | Cl 95% | P | Plac Chi | HR | P | Cl 95% | |
| LVI+ | 42,84 | 0,0779 | -1,65 do -0,89 | 0,000 | 43,58 | 0,061 | 0,000 | -3,61 do-1,96 |
The results indicate that both univariate and multivariate Cox models show statistically significant differences in survival between groups, with a lower risk of event in the comparison group. There was a correlation between LVI and the target gene panel. The proportion of mutations was as follows: PTEN 49%, PIK3CA 35%, KRAS 25%, TP53 20%, FGFR-2 14%, CTNNB1 12%, FBXW7 9%, ATM 1%, ALK1 1% and APC 1%. An attempt to combine EMT features with high-grade mutations gave the following results: TP53, KRAS vs. vimentin, E-cadherin and tumor budding ( p > 0.05). Lymphatic invasion was more frequently observed in tumors with TP53 mutation (R = 0.3138, p = 0.009) [5,6]
Opposite results were obtained with respect to FGFR-2 mutations. Here the following results were recorded: LVI (R = 0.2181, p = 0.069), vimentin expression (R = 0.8199, p = 0.000), tumor budding (R = 0.6407, p = 0.000) and absence of E-cadherin (R = 0.6948, p = 0.000).
Discussion
The tumor hallmark of lymphovascular invasion (LVSI) was observed more frequently in high-grade TP53 mutation-positive tumors, particularly in EMT. Our results unanimously confirm that EMT features are useful for prediction, but only LVSI achieved predictive validity in the multivariate Cox model. The approach to elucidating the mechanism of LVI led us to the TP53 pathway, but the FGFR2 mutation in this area is still unclear [5,6] To specifically investigate the clinical utility of lymphatic vessel invasion (LVSI) in endometrial cancer (EC), we analyzed patients with EC, differentiating them according to the level of estrogen receptor (ER) expression. According to the Bokhmann criterion, we identified EC patients with positive estrogen receptor expression (ER+), consistent with type 1 EC [5,6].
Our results showed that LVSI had significant associations with progesterone receptor (PR) positivity in type 1 EC patients and Ki67 and PR positivity in type 2 EC patients who were estrogen receptor negative (ER-). Compared with type 1 EC, we found that LVSI is more common in type 2 EC. LVSI in type 1 EC was also associated with tumor biomarkers such as CA125 and ROMA, with the main effect appearing to be CA125. Additionally, in addition to the Ki67 score, LVSI in type 2 EC was related to tissue factor (TC) [7].
Our results suggest that CA125, acting as a serum tumor biomarker, may play a key role in identifying LVSI in type 1 EC. Importantly, differences in metabolic phenotype may influence the occurrence of LVSI in different EC subtypes. [7].
The prognosis of patients with early endometrial cancer (EC) is generally favorable, but the situation changes significantly in the case of invasion and metastasis, where the prognosis becomes poor [8]. Attention should be paid to lymphatic vascular invasion (LVSI) as it is a clinicopathological feature, closely related to the presence of lymph node metastases, progression-free survival and overall survival in EC [9]
At the same time, there are studies indicating that metabolic disorders may increase the risk of cancer development and progression in EC. Case-control studies conducted in China provide information on the association between EC and levels of total cholesterol, triglycerides (TG), low-density lipoprotein cholesterol, and dyslipidemia. Additionally, there are positive associations with total cholesterol and TG, as well as a negative association with high-density lipoprotein (HDL) levels. It is also interesting that women with type 1 EC have an increased risk, especially those with a BMI ≥ 24.58 compared to women with a lower BMI (< 24.58) [10]
In overweight individuals, a significant increase in lipid biosynthesis and lipid peroxidation is observed. Studies in mouse models show that obesity promotes the invasiveness of endometrial cancer [11]. Yang et al. conducted experiments on Ishikawa cells, exposing them to high levels of glucose, which led to an observed increase in the progression of endometrial cancer. Simon et al. suggest that reducing cardiovascular risk factors, including high cholesterol levels, may significantly reduce the incidence of endometrial cancer over a ten-year follow-up period.[12] Estrogen receptor (ER), an important prognostic factor in hormone-dependent cancers, has a strong association with lymphatic vessel invasion (LVSI). Positive ER expression has been shown to be a beneficial factor for LVSI in endometrial cancer (EC), prompting the division of ECs based on ER expression. Our study found that the incidence of LVSI in type 2 EC was higher (17.60%) than in type 1 EC (6.18%). Jaishankar et al. also noted that significant LVSI is often present in type 2 EC, predicting the presence of lymphatic metastasis and worse clinical outcomes [13].
An interesting finding of our study was the finding that the independent risk factors associated with LVSI differed between EC type 1 and EC type 2. In EC type 1, fasting blood glucose (FBG) and apolipoprotein B (Apo B) were found to be independent risk factors LVSI, while total cholesterol (TC) was a significant risk factor for LVSI in EC type 2. These results suggest an important role of glucose metabolism in LVSI in EC type 1, where endometrioid adenocarcinoma predominates. Dai et al. also noted that diabetes is more common in patients with endometrial adenocarcinoma and positive LVSI. Furthermore, excess body weight was associated with both EC type 1 and EC type 2, depending on body weight, with a stronger association observed for EC type 1 [13,14].
On the other hand, the important role of lipid metabolism in the context of lymphovascular space invasion (LVSI) should be emphasized in the case of endometrial cancer (EC) type 2, mainly characterized by the presence of non-endometrioid adenocarcinoma. Studies have repeatedly confirmed a strong association between cholesterol levels and metastasis in hormone-dependent cancers such as prostate cancer, breast cancer, and EC [15,16]. Additionally, a study by Kho et al. using Mendelian analysis indicated an association between the levels of three blood lipids and the risk of EC in a large population. The team suggests that increasing serum LDL levels may lower the risk of non-endometrioid adenocarcinoma, while increasing HDL levels may increase the risk. Importantly, LDL and HDL play a key role in the progression of non-endometrioid adenocarcinoma [17,18,19].

 ENGLISH
ENGLISH